Rep:Mod:DPC18
David Coogan IMM2 Wiki Page - CID: 01507872
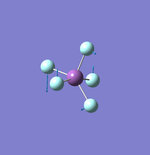
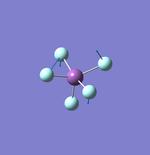
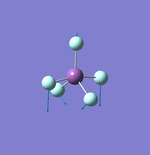
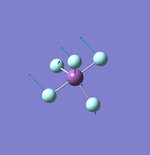
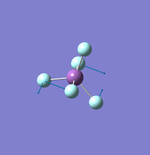
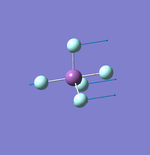
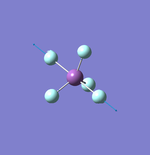
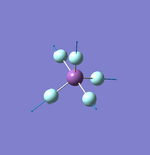
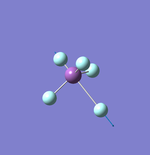
NH3
NH3 Optimisation Information and Results Summary
Molecule: NH3
Type of Calculation: OPTF/FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy E(RB3LYP): -56.5577687 a.u.
RMS Gradient: 4.85 x 10-6 a.u.
Point Group: C3v
N-H Bond Distance: 1.02 Å
H-N-H Bond Angle: 106°
Final Optimisation Table:
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986278D-10
Optimization completed.
-- Stationary point found.
File: The optimisation file is liked to here
JMOL File:
NH Optimised File |
NH3 Frequency Analysis
Display Vibrations:
| Wavenumber
(cm-1 ) |
1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
|---|---|---|---|---|---|---|
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity
(arbitrary units) |
145 | 14 | 14 | 1 | 0 | 0 |
| Image | 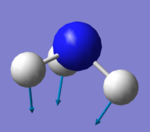 |
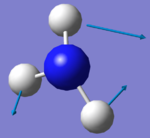 |
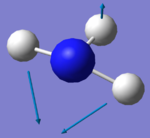 |
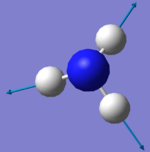 |
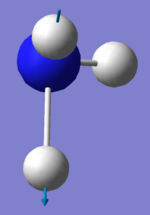 |
|

How many modes do you expect from the 3N-6 rule?
Non Linear Molecule: (3*4)-6 = 6 Vibrational Modes Expected
Which modes are degenerate (i.e. have the same energy)?
There are two sets of degenerate modes. Modes 2 & 3 are degenerate and modes 5&6 are degenerate.
Which modes are "bending" vibrations and which are "bond stretch" vibrations?
Modes 1,2 and 3 are bending vibrations whereas modes 4, 5 and 6 are bond stretches.
Which mode is highly symmetric?
Modes 1 and 4 are highly symmetric. They both have a symmetry of A1.
One mode is known as the "umbrella" mode, which one is this?
Mode 1 is the umbrella mode.
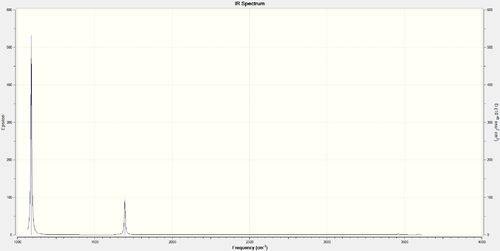
How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
You would expect to see 2 bands, each of decreasing size as you increase the wavelength. There are also 2 small blips at very high wavelengths but these are negligible.
NH3 Charge Analysis
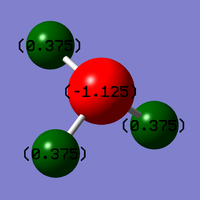
Nitrogen is a larger, more electronegative atom and as such you would expect it to have a more negative charge when compared to Hydrogen. From the values calculated it can be seen that this is the case:
Charge on Nitrogen: -1.13 D
Charge on Hydrogen's: 0.34 D
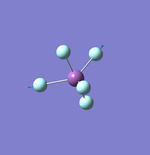
N2
N2 Optimisation Information and Results Summary
Molecule: N2
Type of Calculation: OPTF/FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy E(RB3LYP): -109.5241287 a.u.
RMS Gradient: 6 x 10-7 a.u.
Point Group: D∞h
N-N Bond Distance: 1.11 Å
N-N Bond Angle: 180°
Final Optimisation Table:
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401031D-13
Optimization completed.
-- Stationary point found.
File: The optimisation file is linked to here
JMOL File:
N Optimised File |
Although the JMOL file only shows a single bond the optimised model shown below confirms it is a triple bond. This is also confirmed by the fact that the energy is much more negative than would be expected for a single bond. It is likely due to the modeling software used that the bond shown is single.

N2 Frequency Analysis
Display Vibrations:
| Wavenumber (cm-1 ) | 2457 |
|---|---|
| Symmetry | D∞h |
| Intensity (arbitrary units) | 0 |
| Image | 
|

Using the 3N-5 rule for two atoms it would be expected that there is only one vibrational mode, which is true. For a molecule to be IR active there must be a change in dipole moment. Because N2 is a homonuclear linear molecule there is no change in dipole moment and so it does not appear on an IR spectra. N2 has a single stretching frequency.
N2 Charge Analysis
N2 is a homonuclear linear molecule and so has an equal charge distribution with no dipole.
Charge on Nitrogen's: 0 D

N2 Structure and Reactivity
Mono-metallic TM complex that coordinates N2
I found the structure with the identifier BARTOF whose full name is (dinitrogen)-(2,6-bis(1-(2,6-diisopropylphenylimino)propyl)pyridine)-iron. The N-N Bond Distance is 1.11 Å.
File: The structure file is linked to [1]
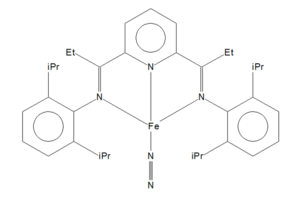
The bond distance found computationally was shorter than that of the experimental molecule. This is due to a number of factors. Firstly, experimentally, the N2 is bonded to an Fe atom, which pulls electron density away from the N-N bond, reducing bond strength and therefore making the bond longer. There are also computational factors involved. Computational models are modelled in the gas phase where experimentally it is done in solid state. This change will have an error associated with it causing a difference between the two bond length values. Finally, the method that we used to optimise the structure may also explain why there is a difference; had we used another optimisation method the difference between the variations of the bond lengths may change.
In the journal article that this structure was reported in it was shown that this compound, (iPrPDI)FeN2, was in equilibrium with a similar compound where the Fe had five coordinate bonds (the fifth being with another N2 group). A combination of spectroscopic and computational analysis showed the electronic structures of these two species differs. In this experiment the ORCA programming package was used. Though the same method was used (B3LYP), a different basis set was used. In our calculations the N-Fe-N bond angle calculated was 163.3°, whereas the report stated a value 155.8°. These differences used to calculate the angle may account for differences between our computationally calculated values and those of the experimental calculated values.[1]
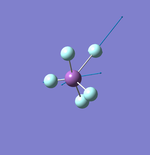
H2
H2 Optimisation Information and Results Summary
Molecule: H2
Type of Calculation: OPTF/FREQ
Calculation Method: RB3LYP
Basis Set: 6-31G(d,p)
Final Energy E(RB3LYP): -1.1785394 a.u.
RMS Gradient: 1.7 x 10-7 a.u.
Point Group: D∞h
H-H Bond Distance: 0.74 Å
H-H Bond Angle: 180°
Final Optimisation Table:
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
File: The optimisation file is linked to here
JMOL File:
N Optimised File |
H2 Frequency Analysis
Display Vibrations:
| Wavenumber (cm-1 ) | 4466 |
|---|---|
| Symmetry | D∞h |
| Intensity (arbitrary units) | 0 |
| Image | 
|

As for N2, we use the 3N-5 rule again for two atoms to see that it is expected that there is only one vibrational mode, which is again true. Once again, for a molecule to be IR active there must be a change in dipole moment. Because H2 is a homonuclear linear molecule there is no change in dipole moment and so it does not appear on an IR spectra. H2 has a single stretching frequency at a higher frequency than N2.
H2 Charge Analysis
H2 is also a homonuclear linear molecule and so has an equal charge distribution with no dipole.
Charge on Hydrogen's: 0 D

H2 Structure and Reactivity
Mono-metallic TM complex that coordinates H2
I found the structure with the identifier LEYCOH whose full name is Dihydrido-tricarbonyl-bis(tri-isopropylphosphine)-chromium. The H-H Bond Distance is 0.67 Å.
File: The structure file is linked to [2]
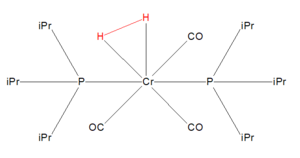
Once again, the bond distance found computationally was shorter than that of the experimental molecule. This is likely due to experimental factors as well as computational limitations as listed above for the N2 Coordination Compound.
The Haber-Bosch Process
The Haber-Bosch Process is: N2 + 3H2 → 2NH3.
We can determine the energy for this reaction by using the following data:
E(NH3): -56.5577687 a.u.
2 x E(NH3): -113.1155374 a.u.
E(N2): -109.5241287 a.u.
E(H2): -1.1785394 a.u.
3 x E(H2): -3.5356182 a.u.
ΔE = 2xE(NH3) - [E(N2) + 3xE(H2)]:
= -113.1155374 - [-109.5241287 + -3.5356182] = -113.1155374 - -113.0597469 = -113.1155374 + 113.0597469 = -0.0557905 a.u. 1 a.u. = 2625.5 kJ mol-1 -0.0557905 a.u. = -146.47796891 kJ mol-1
ΔE = -146.5 kJ mol-1
The combined energies of hydrogen and nitrogen have a higher energy than that of the ammonia product. The energy change for the reaction is negative meaning it is an exothermic reaction and so ammonia is a more stable than the gaseous reactants.
Project Molecule: SbF5
SbF5 Optimisation Information and Results Summary
Molecule: SbF5
Type of Calculation: OPTF/FREQ
Calculation Method: RB3LYP
Basis Set: LanL2DZ
Final Energy E(RB3LYP): -504.72072892 a.u.
RMS Gradient: 8.998 x 10-5 a.u.
Point Group: D3h
Sb-F Bond Distance: 1.90 Å / 1.91 Å
F-Sb-F Bond Angle: 90°/120°/180°
Final Optimisation Table:
Item Value Threshold Converged?
Maximum Force 0.000179 0.000450 YES
RMS Force 0.000080 0.000300 YES
Maximum Displacement 0.000741 0.001800 YES
RMS Displacement 0.000335 0.001200 YES
Predicted change in Energy=-3.064162D-07
Optimization completed.
-- Stationary point found.
File: The optimisation file is liked to here
JMOL File:
NH Optimised File |
SbF5 Frequency Analysis
Display Vibrations:

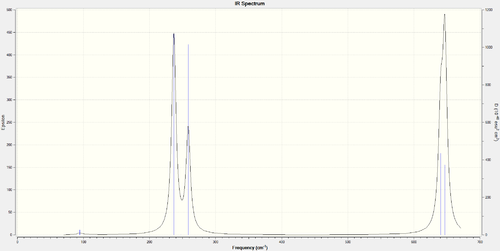
SbF5 Charge Analysis
Fluorine is the most electronegative atom and so has a negative charge. When bonded to Antimony there is a dipole moment from the centre outwards. The fluorine's with the slightly longer bond length have a more negative charge. From the values calculated it can be seen that this is the case:
Charge on Antimony: 3.04 D
Charge on Fluorine's: -0.61 D

SbF5 Molecular Orbitals
References
- ↑ P. J. Chirik, S. DeBeer, Z. R. Turner, K. D. Finkelstein, J. M. Hoyt, C. Milsmann, K. Wieghardt and S. C. E. Stieber, Inorg. Chem., 2012, 51, 3770–3785.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have recieved your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 1/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES
It is worth noting that the size of the peaks is related to the intensity values in the Infrared column of the display vibrations table. The smaller peaks are from the vibrations with a low intensity and these vibrations are those which do not change the dipole moment of the molecule.
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES - good explanation of the IR spectra.
However you have given a bond angle of 180 for N2 and H2, there are no bond angles in diatomic molecules. Bond angles involve exactly 3 atoms.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 5/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES - good explanation of the MOs.
Independence 1/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or
YES - you found a crystal structure for H2 coordinated to a metal complex.
Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far