Rep:Mod:DMD216
Dat's Wiki Page
NH3 molecule
Summary information
| N-H bond distance | 1.01798 |
| H-N-H bond angle | 105.741 |
| Calculation method | B3LYP |
| Basis set | 6-31G(d,p) |
| Final energy E(RB3LYP) in au | -56.55776873 |
| RMS gradient | 0.00000485 |
| Point Group | C3V |
Optimisation
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986269D-10
Optimization completed.
-- Stationary point found.
NH3 |
The optimisation file is linked to here
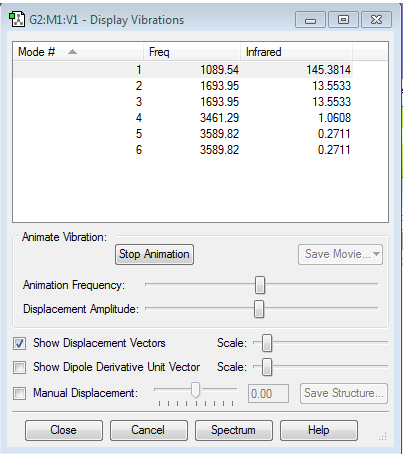
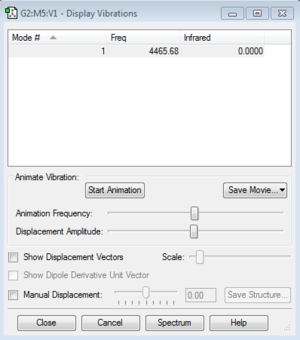
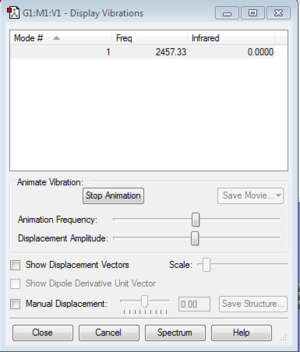
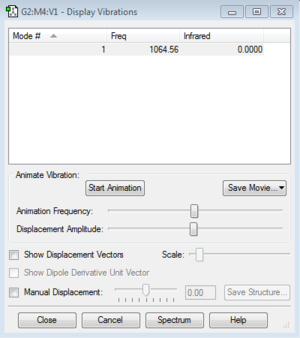
Vibrations and charges of NH3
How many modes do you expect from the 3N-6 rule? 6 Which modes are degenerate (ie have the same energy)? 2 and 3, 5 and 6 Which modes are "bending" vibrations and which are "bond stretch" vibrations? 1,2 and 3 are bending vibrations, 4,5 and 6 are bond stretch vibrations Which mode is highly symmetric? 4 One mode is known as the "umbrella" mode, which one is this? 1 How many bands would you expect to see in an experimental spectrum of gaseous ammonia? 2 bands because there are two large peaks, but the other peaks are too small relative to the larger peaks so are not counted.
Charge Distribution
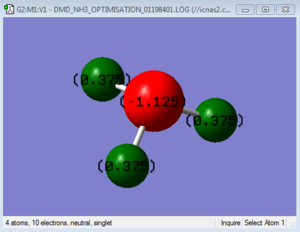
H2 Molecule
Summary information
| H-H bond distance | 0.74279 |
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy E(RB3LYP) in au | -1.17853936 |
| RMS gradient | 0.00000017 |
| Point Group | D∞h |
Optimisation
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
H2 |
The optimisation file is linked to here
N2 Molecule
Summary information
| N-N bond distance | 1.10550 |
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy E(RB3LYP) in au | -109.52412868 |
| RMS gradient | 0.00000365 |
| Point Group | D∞h |
Optimisation
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.401029D-13
Optimization completed.
-- Stationary point found.
N2 |
The optimisation file is linked to here
Haber-Bosch process
N2 + 3H2 --> 2NH3
E(NH3)= -56.55776873 2*E(NH3)= -113.1155375 E(N2)= -109.52412868 E(H2)= -1.17853936 3*E(H2)= -3.53561808 ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579074 au ΔE= -146.478599028 kJ/mol ΔE= -146.48 kJ/mol (2dp)
The ammonia product is the most stable as the formation of ammonia is a exothermic reaction so energy is released to form a more stable product.
The literature value for the formation of ammonia in the Haber Bosch process is -91.2kJ/mol.[1] This literature value is very different from the calculated value because the literature value is experimentally calculated under standard conditions such as 298K. The computer calculated energies are calculated under conditions assumed to be for example 0K. Therefore the energy values from the computer are very different from the experimentally calculated enthalpies.
F2 molecule
Summary information
| F-F bond distance | 1.40298 |
| Calculation method | RB3LYP |
| Basis set | 6-31G(d,p) |
| Final energy E(RB3LYP) in au | -199.49825220 |
| RMS gradient | 0.00000069 |
| Point Group | D∞h |
The literature value of the F-F bond length is 1.41 angstroms .[2] This literature value is very close to the calculated F-F bond length because the computer can calculate the first derivative of the potential energy surface which provides us of the structure we want.
Optimisation
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000001 0.001800 YES
RMS Displacement 0.000002 0.001200 YES
Predicted change in Energy=-1.747692D-12
Optimization completed.
-- Stationary point found.
F2 |
The optimisation file is linked to here
F2 is a fully symmetrical molecule and is linear and so has an equal charge distribution.