Rep:Mod:DKW2845
NH3 molecule
Calculation method: B3LYP
Basis set:6-31G(d,p)
Final energy E(RB3LYP) = -56.44397188 a.u.
RMS gradient = 0.05399560 a.u.
Point group = C3V
Bond distance(N-H) = 1.01798 angstroms
Bond angle(H-N-H) = 105.741 degrees
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 molecule optimised |
The optimisation file is liked to here
| Number | wavenumber | Symmetry | Intensity | Image |
|---|---|---|---|---|
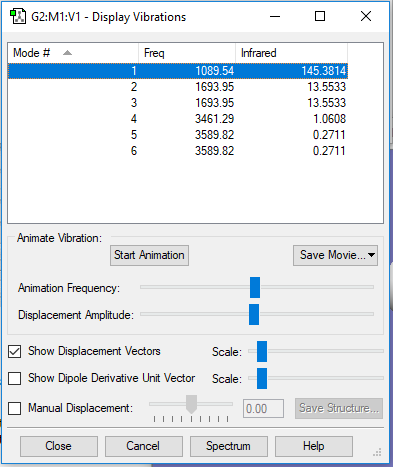
| 1 | 1090 | A1 | 145 | 
|
| 2 | 1694 | E | 14 | 
|
| 3 | 1694 | E | 14 | 
|
| 4 | 3461 | A1 | 1 | 
|
| 5 | 3590 | E | 0 | 
|
| 6 | 3590 | E | 0 | 
|
1 2 3
A1 E E
Frequencies -- 1089.5366 1693.9474 1693.9474
Red. masses -- 1.1800 1.0644 1.0644
Frc consts -- 0.8253 1.7996 1.7996
IR Inten -- 145.3814 13.5533 13.5533
Atom AN X Y Z X Y Z X Y Z
1 7 0.00 0.00 0.12 -0.07 0.00 0.00 0.00 0.07 0.00
2 1 0.00 -0.21 -0.53 0.76 0.00 0.00 0.00 0.15 0.26
3 1 0.18 0.11 -0.53 0.08 -0.39 0.22 0.39 -0.53 -0.13
4 1 -0.18 0.11 -0.53 0.08 0.39 -0.22 -0.39 -0.53 -0.13
4 5 6
A1 E E
Frequencies -- 3461.2932 3589.8170 3589.8170
Red. masses -- 1.0272 1.0883 1.0883
Frc consts -- 7.2510 8.2634 8.2634
IR Inten -- 1.0608 0.2711 0.2711
Answers
1. 6 modes are expected from 3N-6 rule
2. the modes with frequencies 1694 and 3590 are degenerate
3. The modes with frequencies 1090, 1694, and 1694 are "bending" vibrations and the modes with frequencies 3461, 3590, and 3590 are "bond stretch" vibrations
4. The modes with frequencies 1090 and 3461 are highly symmetric.
5. The mode with frequency 1090 is the "umbrella" mode.
6. I would expect 4 bands in an experienmental spectrum of ammonia gas.
NBO charge distribution
charge on the central N atom = - 1.125
charge on the 3 H atoms = + 0.375
I expect the N atom to have negative charge and the H atoms to have eqaul and opposite charges because N atom is more electronegative than the H atoms so the electron in the N-H bond will be located closer to the N atom than the H atom which gives it negative charge, the H atoms will have equal positve charge as their positions to the N atom are equivalent and the electrons move away from them and leaving their charge positive.
N2 molecule
Calculation method: B3LYP
Basis set: 6-31G(d,p)
Final energy E(RB3LYP) = -109.52412868 a.u.
RMS gradienbt = 0.00000060 a.u.
Point group = DinfH
bond distance(NN) = 1.10550 angstroms
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
N2 molecule optimised |
The optimisation file is liked to here
| Number | Wavenumber | Symmetry | Intensity | Image |
|---|---|---|---|---|
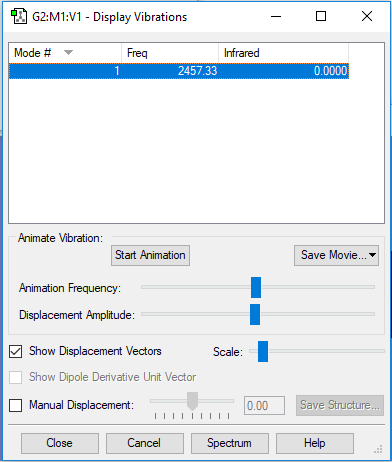
| 1 | 2457 | SGG | 0 | 
|
NBO charges: 0 on N1 atom and 0 on N2 atom
H2 molecule
Calculation method: RB3LYP
Basis set: 6-31G(d,p)
Final energy E(RB3LYP) = -1.17853936 a.u.
RMS Gradient Norm = 0.00000017 a.u.
Point Group = D*H
Bond length(H-H) = 0.74279 angstroms
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 0.7428 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
H2 molecule optimised |
The optimisation file is liked to here
| Number | Wavenumber | Symmetry | Intensity | Image |
|---|---|---|---|---|
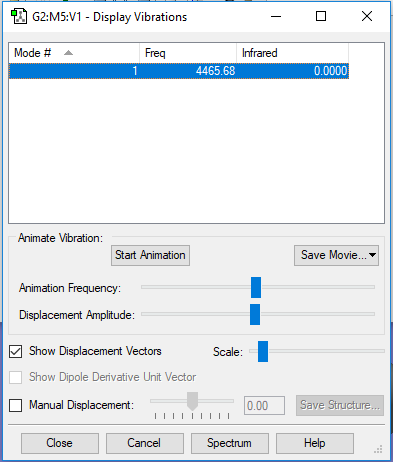
| 1 | 4466 | SGG | 0 | 
|
NBO charges: 0.000 on both H atoms.
Monometallic transition metal complex
Unique identifier: EQEPUN
Bond distance(NN) = 1.055 angstroms
The NN bond distance in the crystal structure is shorter than the NN bond distance in the computational distance. The NN bond is shorter in the crystal structure as in the transition metal complex is due to the back-bonding effect which increases the electron density between the two N atoms and therefore bond order, so the bond distance decreases.
Energy calculations
E(NH3) = -56.55776873 a.u.
2*E(NH3) = -113.1155375 a.u.
E(N2) = -109.52412868 a.u.
E(H2) = -1.17853936 a.u.
3*E(H2) = -3.53561808 a.u.
delta E = 2*E(NH3) - (E(N2) + 3*E(H2)) = -0.05579074 a.u.
delta E = -0.05579074*2625.5 = -146.45 kJ/mol
The ammonia product is more stable.
SbF5 molecule
Calculation method: RB3LYP
Basis set: LANL2DZ
Final energy E(RB3LYP) = -504.72005611 a.u.
Point group = D3H
Bond distance (Sb-F) equatorial = 1.89632 angstroms
Bond distance (Sb-F) axial = 1.90898 anstroms
Bond angle (F-Sb-F) equatorial = 120.000 degrees
Bond angle (F-Sb-F) axial-equatorial = 90.000 degrees
Bond angle (F-Sb-F) axial-axial = 180.000 degrees
Item Value Threshold Converged?
Maximum Force 0.000143 0.000450 YES
RMS Force 0.000059 0.000300 YES
Maximum Displacement 0.000589 0.001800 YES
RMS Displacement 0.000248 0.001200 YES
Predicted change in Energy=-1.693508D-07
Optimization completed.
-- Stationary point found.
----------------------------
! Optimized Parameters !
! (Angstroms and Degrees) !
-------------------------- --------------------------
! Name Definition Value Derivative Info. !
--------------------------------------------------------------------------------
! R1 R(1,2) 1.909 -DE/DX = -0.0001 !
! R2 R(1,3) 1.8963 -DE/DX = 0.0001 !
! R3 R(1,4) 1.8963 -DE/DX = 0.0001 !
! R4 R(1,5) 1.8963 -DE/DX = 0.0001 !
! R5 R(1,6) 1.909 -DE/DX = -0.0001 !
! A1 A(2,1,3) 90.0 -DE/DX = 0.0 !
! A2 A(2,1,4) 90.0 -DE/DX = 0.0 !
! A3 A(2,1,5) 90.0 -DE/DX = 0.0 !
! A4 A(3,1,4) 120.0 -DE/DX = 0.0 !
! A5 A(3,1,5) 120.0 -DE/DX = 0.0 !
! A6 A(3,1,6) 90.0 -DE/DX = 0.0 !
! A7 A(4,1,5) 120.0 -DE/DX = 0.0 !
! A8 A(4,1,6) 90.0 -DE/DX = 0.0 !
! A9 A(5,1,6) 90.0 -DE/DX = 0.0 !
! A10 L(2,1,6,3,-1) 180.0 -DE/DX = 0.0 !
! A11 L(2,1,6,3,-2) 180.0 -DE/DX = 0.0 !
! D1 D(2,1,4,3) 90.0 -DE/DX = 0.0 !
! D2 D(2,1,5,3) -90.0 -DE/DX = 0.0 !
! D3 D(2,1,5,4) 90.0 -DE/DX = 0.0 !
! D4 D(3,1,5,4) 180.0 -DE/DX = 0.0 !
! D5 D(3,1,6,4) -120.0 -DE/DX = 0.0 !
! D6 D(3,1,6,5) 120.0 -DE/DX = 0.0 !
! D7 D(4,1,6,5) -120.0 -DE/DX = 0.0 !
--------------------------------------------------------------------------------
SbF5 molecule optimised |
The optimisation file is liked to here
NBO charge distribution:
Charge on central Sb atom = + 3.040
Charge on equatorial F atoms = - 0.606
charge on axial F atoms = - 0.612
Molecular orbitals
1.
This orbital has energy of -0.48226 a.u.. It is the three non-bonding p orbital on the equatorial F atoms. They are non-bonding as there are no interactions between the orbitals. It has a C3 axis, one horizontal mirror plane and three vertical mirror plane. It is slightly lower in energy than the HOMO. I can also see that there are three vertical mirror planes for this MO.
2.
This is likely to be the lowest energy bonding MO and possibly have AO conbtributions from F 2s AOs and Sb 2s AO as I see that it has sperical shape around the molecule and the electron density is positive as the color of the orbital is all red. It has energy of -1.27228 a.u..
3.
This MO has energy of -24.77133a.u.. It is possibly come from 1s AOs from the equatorial F atoms as the MO is very deep in energy. I can also see on the graph that there is very little electron density on the F atoms, probably because the electrons are too deep in energy to be involved in any kind of bonding.
4.
This MO has energy of -1.23152 a.u.. It is possibly the anti-bonding orbitals on the axial F atoms. This MO is probably come from the 2p electrons on the F atom as it have a dumbbell shape. The graph shows that it has a horizontal nodal plane and the electron density is positive above and negative below.
5.
This is the LUMO of the SbF5 molecule. It has energy of -0.21072 a.u.. This MO is likely to be non-bonding MO as there is no interaction between the orbitals. The MO is likely to be contributed from 4s/5s orbital from Sb atom and 3p orbital from the 5 F atoms.
Marking
Note: All grades and comments are provisional and subjecct to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have recieved your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES - you could have included more subheadings
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 0.5/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - only 2 bands are expected in the real spectrum. The intensities of the stretching vibrations are too low to be seen.
N2 and H2 0.5/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES, you could have explained that the charges are 0 as the electronegativities are equal.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 4.5/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
You have done a good job of presenting this information, well done! You could have explained the charges using an electronegativity argument. The description of MO5 is wrong. Due to the out of phase interactions of the AOs this MO resembles an anti-bonding Orbital.
Independence 0/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or
Do some deeper analysis on your results so far
NO