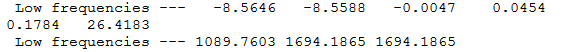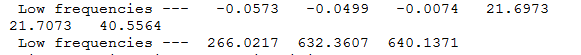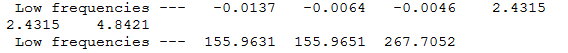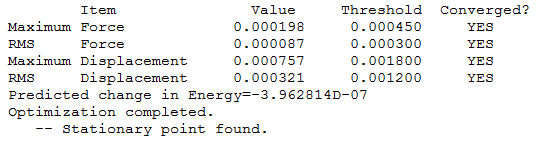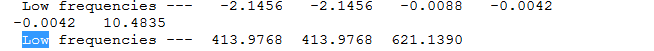Rep:Mod:DJN160518
BH3 Section
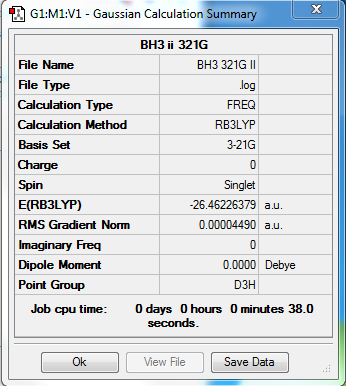
BH3
B3LYP/3-21G level
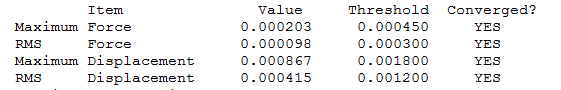
Convergence information BH3
Frequency analysis log file BH3
File:BH3 631G SUMDAT.PNG
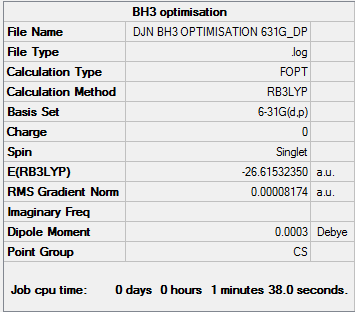
B3LYP/6-31G level
Convergence information BH3
Frequency analysis log file BH3
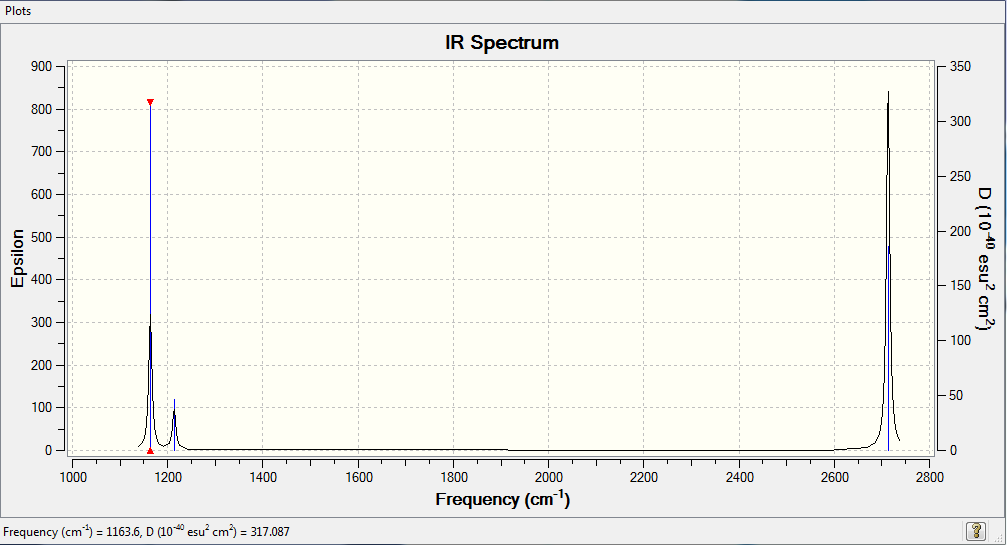
IR SPECTRA BH3
We only see 3 peaks in our spectra despite 6 vibrational modes because several modes are degenerate and 1 of the stretches is symmetrical so there is no change in dipole moment therefore we see no peak in the IR.
Ng611 (talk) 18:37, 30 May 2018 (BST) How many degenerate sets are there, and which ones are they? Which stretch has no change in dipole moment?
Ng611 (talk) 18:38, 30 May 2018 (BST) You needed to tabulate these yourself, including their symmetry labels and assignments.
Optimised molecule 6-31G
BH3 |
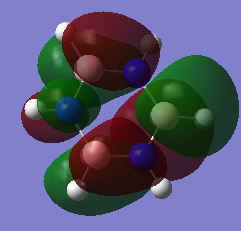
MOs for BH3
Real MOs vs Our MOs:
Are there any significant differences between the real and LCAO MOs?
What does this say about the accuracy and usefulness of qualitative MO theory?
There are no major differences between the real MOs and the LCAO MO's meaning qualitative MO theory is certainly useful as we can see great similarity between our computational analysis and the qualitatively constructed MOs.
Ng611 (talk) 18:39, 30 May 2018 (BST) Good MO analysis. Could you see any differences at all?
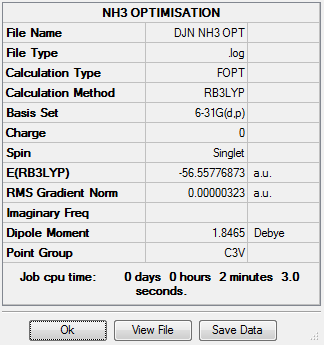
NH3
RB3LYP, 6-31G(d,p)
NH3 summary info
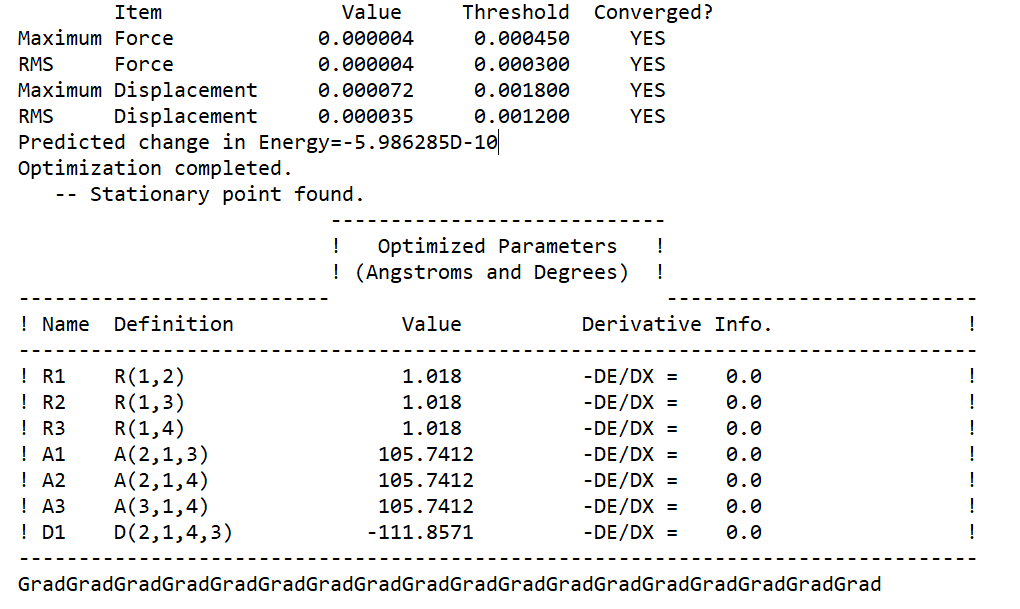
NH3 convergence info
NH3 frequency info
NH3 molecule
NH3 |
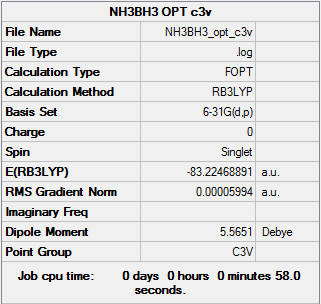
NH3BH3
RB3LYP, 6-31G(d,p)
NH3BH3 summary info
NH3BH3 convergence info
NH3BH3 frequency info
NH3BH3 molecule
NH3BH3 |
Calculations
E(NH3)=-56.55776873 a.u.
E(BH3)=-26.61532350 a.u.
E(NH3BH3)=-83.22468891 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
ΔE= (-83.22468891 a.u.) - ((-56.55776873 a.u.) + (-26.61532350 a.u.))
Bond Dissociation Energy (N-B)=-0.05159668 a.u.
Bond Association Energy (N-B)=-0.516 a.u. ≡ 135 kJmol-1
N-B is a relatively weak, comparatively O-H bond dissociation value [1] = 458.9 kJmol-1
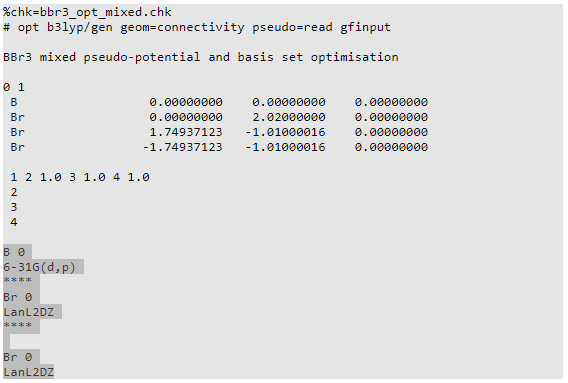
BBr3
RB3LYP, GEN
Ng611 (talk) 18:42, 30 May 2018 (BST) Be careful here as Gen is not a valid basis set descriptor.
BBr3 basis set info
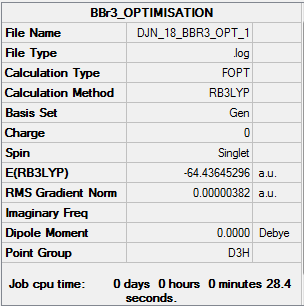
BBr3 summary info
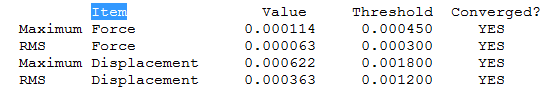
BBr3 convergence info
BBr3 frequency info
BBr3 molecule
Ng611 (talk) 18:42, 30 May 2018 (BST) You also need a d-space link for this molecule.
BBr3 |
Project section: Aromaticity
Benzene
RB3LYP, 6-31G(d,p)
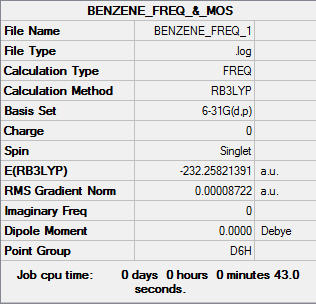
Benzene summary info
Benzene convergence info
Benzene frequency info
File frequency data: Media:BENZENE FREQ 1.LOG
Benzene
Benzene |
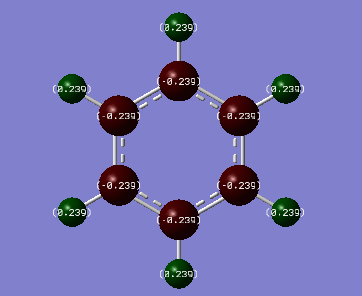
Benzene Charge Distribution:
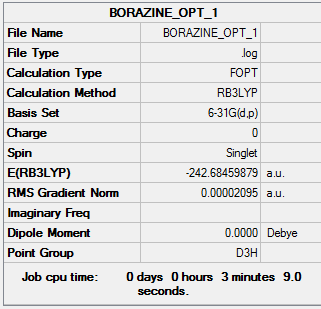
BORAZINE
RB3LYP, 6-31G(d,p)
Borazine summary info
Borazine convergence info
Borazine frequency info
File frequency data: Media:BORAZINE FREQ 2.LOG
Borazine
Borazine |
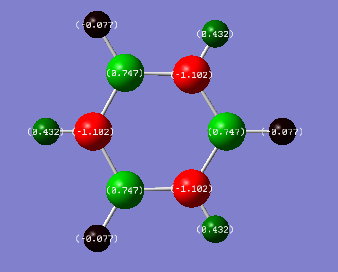
Borazine Charge Distribution:
Charge distribution:Benzene vs Borazine
| Benzene | Borazine |
|---|---|
 |
|
Colour range used:[-1.102,1.102]
| C | H | B | N |
|---|---|---|---|
| 2.55 | 2.20 | 2.04 | 3.04 |
Benzene explanation
On Benzene we can see that charge is more densely located on the negative looking C atoms (-0.239, red) than for the more positive H atoms (0.239, green). C atoms are more electronegative than H atoms (2.55 vs 2.20 using the Pauling scale), they have a greater tendency to attract electron density towards themselves in a covalent bond. Also benzene has a delocalised π ring system of electrons from a LCAO σ framework formed from sp2 hybridised carbons which further implies the higher charge density on the carbon atoms
Borazine explanation
Borazine is slightly more complicated than Benzene. The bright red N atoms are the most negative, -1.102, thus have the highest electron density. This is beacuse they have the greatest electronegativity (3.04). The (bright green) H atoms bonded to the N are very positive (0.432) as most of the electron density is located on the N, the difference in electronegativity between N and H is relatively large (Δχ=|3.04-2.20|=0.84) further implying these charge density values for N and H. The electronegativities of B and H are very similar, 2.04 and 2.20 respectively. H bonded to B (dark red) is slightly more electronegative hence we see these H atoms have a higher electron density indicated by a charge density of -0.077. Thus B has a charge density of 0.747.
Ng611 (talk) 18:43, 30 May 2018 (BST) Good discussion of the effects of electronegativity on the overall charge distribution. What do the partial charges sum to, and is there any difference in partial charge for atoms related by symmetry?
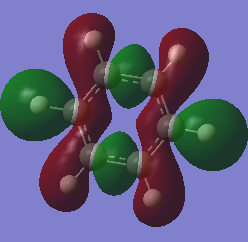
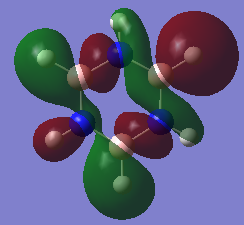
MOs: benzene vs Borazine
Ng611 (talk) 18:45, 30 May 2018 (BST) Well done for comparing the correct MOs by shape and not energtic ordering (which is not necessarily reliable). I would include a brief discussion of the overall symmetry and differences between the molecules and how these relate to differences in the MOs to improve this section further. Perhaps also consider discussing the constituent AOs that form the MOs and the overall symmetry of the MO.
Aromaticity
Huckel's rules for determining aromaticity:
1) (4n + 2)π (n is a non-negative integer) 2) The molecule is cyclic 3) The molecule is planar 4) The molecule is fully conjugated i.e. (p-orbitals at every atom in the ring) (side note: 4nπ = Antiaromatic, very unstable) [3]
Benzene can be described by these simple rules and fits the aromatic picture. Huckel's rule can also be used to describe aromatic ions such as a cyclopentadienyl anion. There also exists a three dimensionsal rule using 2(n + 1)2 π-electrons used to classify for example a fullerene.[4]
Huckel's rule breaks down for cyclic compounds more than three fused aromatic nuclei[5] such as pyrene and coronene with 16 and 24 conjugated electrons respectively. these fail the (4n + 2)π electron rule but are aromatic. Further research seems to indicate that defining aromaticity is not as black and white as traditionally thought, and is more of a sliding scale. MOs can be used to help identify aromatic character.
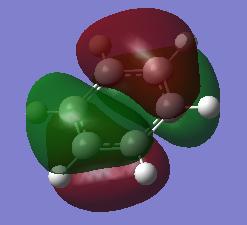
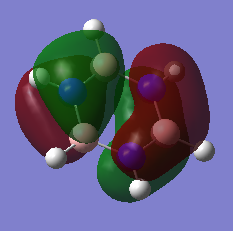
| Benzene MO 17 | Borazine MO 17 |
|---|---|
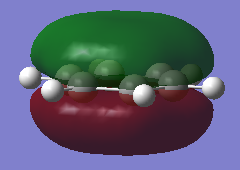 |

|
From MOs 17 for Borazine and Benzene we can see the idea of an electron being delocalised in some electron cloud. This demonstrates a more abstract way of thinking about aromaticity, originating from this basic idea of an electron being able to become delicalised in a ring sysytem rather than the classical, rather restrictive definition.
Ng611 (talk) 18:50, 30 May 2018 (BST) This is a very basic discussion on aromaticity and some more modern perspectives on the topic would improve this section further. Recent research has demonstrated that there is more to aromaticity than pi delocalisation. What you have is absolutely correct, but more discussion is needed.
Ng611 (talk) 18:50, 30 May 2018 (BST) Some very good calculations here. Take care with regards to your layout (e.g.: screencapping blocks of text isn't a great approach) and with regard to the level of detail in your discussion. You have good results from your calculations but you need to analyse them more thoroughly.
References
- ↑ Lehninger, Albert L.; Nelson, David L.; Cox, Michael M. (2005). Lehninger Principles of Biochemistry (4th ed.). W. H. Freeman. p. 48. ISBN 978-0-7167-4339-2. Retrieved May 20, 2016.
- ↑ A.L. Allred, J. Inorg. Nucl. Chem., 1961, 17, 215.
- ↑ Badger, G.M. Aromatic Character and Aromaticity. London, England: Cambridge University Press, 1969.
- ↑ Hirsch, Andreas; Chen, Zhongfang; Jiao, Haijun (2000). "Spherical Aromaticity in Ih Symmetrical Fullerenes: The 2(N+1)2 Rule". Angew. Chem. Int. Ed. Engl. 39 (21): 3915–17.
- ↑ Roberts, John D.; Streitwieser, Andrew, Jr.; Regan, Clare M. (1952). "Small-Ring Compounds. X. Molecular Orbital Calculations of Properties of Some Small-Ring Hydrocarbons and Free Radicals". J. Am. Chem. Soc. 74 (18): 4579–82.