Rep:Mod:DAAO org
Module 1: Structure and Spectroscopy
Molecular modelling has become increasingly important to organic chemistry. It has many applications and has been used to rationalise possible products of a reaction and therefore aid possible changes. The following work shows the diverse ways that molecular modelling can be of use to organic chemists. Firstly, molecular mechanics will be employed to explore three reactions through analysis of either their products, reactants and/or intermediates. These three reactions include the hydrogentation of the cyclopentadiene dimer, the nucleophilic addition to two difference NAD+ analogues and the synthesis of taxol. Semi-empirical molecular orbital theory will then be used to consider the regioselective addition of electrophilic reagents such as dichlorocarbene to both a chlorinated di- and monoalkene. This study of molecular modelling will be finished with a mini-project based on the constitutional isomers 2-Amino-3,5-dinitro-1-phenylthio- and 4-Amino-3,5-dinitro-1-phenylthiobenzenes. This mini-project will involve predicting and analysing both the 13C NMR and IR spectra of both isomers.
Modelling Using Molecular Mechanics
Molecular modelling is used in order to investigate chemical reactivity. This method is used throughout the following exercises however, it must be noted that there are limitations to molecular mechanics. Molecular mechanics is based on compounds that have already been analysed experimentally.This means that it is not suitable for new compounds that may be unusual for example in their bonding.[1] Using the Molecular Mechanics approach, one can ascertain the minimum energy geometry of a molecule by considering its strain, steric repulsion and hydrogen bonding and adjust bonds in order to achieve a molecule with the lowest energy geometry[2] . The ChemBio3D program will be used along with the Allinger MM2 and MMFF94 molecular mechanics models below.
The Hydrogenation of Cyclopentadiene Dimer
In the Diels-Alder cycloaddition of two cyclopentadiene molecules, when approaching the diene, the dienophile may have its substituents facing towards or away from the diene leading to the endo and exo isomers respectively.[3] After optimising the geometries of these isomers in ChemBio3D Ultra using the MM2 force field option, it was seen that the exo product (Molecule 1) is lower in energy by 2.1207 kcal/mol. The exo isomer is therefore the more stable and hence formed under thermodynamic conditions. Looking at Table 1, it can be seen that the Van der Waal’s contribution to the total energy is greater for the endo isomer than that if the exo isomer. This can be explained as there is strain between the hydrogens related in a 1,4 relationship (1,4 strain). The torsion enery term has the greatest difference between the two isomers. This is due to the greater steric strain in the endo isomer. Under kinetic control, formation of the endo isomer (Molecule 2) exclusively has been seen.[4] After examining the transition state of this reaction, R. Sustmann et al. came to the conclusion that the endo addition selectivity is due to the proximity of the two charges of the zwitterion resonance structure of the endo transition state stabilising the transition state better that that of the exo adduct.[4] The formation of the endo isomer can also be attributed to its transition state being stabilised by the favourable bonding interaction or in other words the 'secondary orbital interactions' between between the back of the diene and dienophile.[1] [5]
Hydrogenation of the cyclopentadiene dimer initially only forms one of the two possible hydrogenation products (Molecules 3 and 4). It is only after further hydrogenation that the tetrahydro derivative is formed. Results from optimising the geometry in ChemBio3D and using the MM2 force field option are shown in Table 2. These calculations show that Molecule 4 is lower in energy than Molecule 3 and therefore is the more thermodynamically stable hydrogenation product. For that reason, it can be said that the hydrogenation leading to Molecule 4 is relatively easier by 3.8007 kcal/mol. In this case the greatest diference in energy contribution comes from the bend term.This term is related to the energy involved with the bond stretching from the equilibrium bond angle.[6] Molecule 3 has a greater bend energy due to the strain between the double bond and bridgehead hydrogens which is not seen in Molecule 4. Molecule 4 also has a lower stretch and Van der Waals contributions to the total energy. Other contributions to the total energy not included in the table are the non-Van der Waal forces and stretch-bend interactions.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
The work of A. G. Schultz et al. on the ‘Regio- and Selective Control in the Addition of the Grignard Reagents to the Pyridine Ring System’ show that gringnard reagents are extremely selective of N-methyl and N-benzyl salts of nicotinic acid derivatives such as Molecules 5 and 7.[7]
|
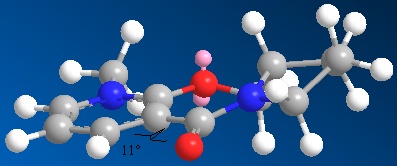
Using ChemBio3D Ultra and the MM2 force field option again, an energy of 43.1367 kcal/mol was achieved after optimisation for Molecule 5 (N-methyl Pyridoxazepinone) with a dihedral angle of 11⁰ between the carbonyl and the pyridinium ring. Manually changing the dihedral angle and the starting conformations of the five- and seven-membered rings showed that at angles greater or smaller than 11⁰ the torsion energy contribution increases leading to a greater total energy.
Experimentation with Molecule 5 showed that due to the rigidity of the seven-membered ring the carbonyl can only be either co-planar to or slightly above the plane of the pyridine ring. It is not possible for the carbonyl to be below the plane of the aromatic ring.[7] This is particularly important when looking at the mechanism of the reaction between Molecule 5 and the Grignard reagent MeMgI. It should be noted here that the force field option MM2 does not have the parameters in order to include magnesium in the calculations and so was left out. To include the grignard reagent in calculations the parameters of the progam would have to be changed
A dihedral angle of 11⁰ minimises the interaction between the oxygen of the carbonyl and the closest hydrogens and therefore reduces strain. This angle also allows the magnesium of the grignard reagent to coordinate to the oxygen of the carbonyl and the methyl of the reagent to have the correct orientation in order to attack carbon 4 from the top face each time in the nucleophilic addition. This gives Molecule 6 with the stereochemistry shown below.[7]

|
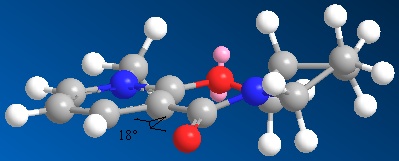
Similar conformation manipulations were done with Molecule 7. It was found that using the MM2 force field option that a dihedral angle of 18⁰ between the carbonyl and the aromatic ring and the bulky iso-propyl and methyl substituents being as far apart as possible gave the lowest energy conformation. Again, varying this angle caused the torsion energy term to increase and therefore the total energy to be greater. Unlike Molecule 5, the carbonyl of Molecule 7 is found below the plane of the pyridinium ring.[7] The nucleophilic addition mechanism is different for Molecule 7 compared to Molecule 5. Here, no coordination is seen, rather the lone pair nitrogen attacks carbon 4 on the top face (thereby avoiding the lone pairs on the carbonyl oxygen) to give the a product with the stereochemistry shown below.
From the conformation manipulations, it was noted that obtaining the lowest geometry is dependent on starting with a similar conformation. One must try different starting geometries and compare the conformations of the located minima in order to ensure a global miniumum has been obtained rather than a local minimum energy.[6]

Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
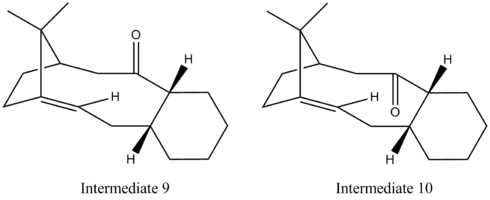
Intermediates 9 and 10 have been suggested as key intermediates in the synthesis of taxol by L. Paquette et al.[8] On standing, atropisomerism is observed due to the lack of rotation about the single bonds.[9] Using ChemBio3D Ultra and the MM2 force field option, the energies of these intermediates were minimised to 54.3973 and 44.3163 kcal/mol for Intermediate 9 and 10 respectively. Intermediate 9 has the carbonyl above the aromatic ring which is in a twist-boat conformation while in Intermediate 10 the carbonyl is below the aromatic ring which has a chair geometry. From the calculations, it was seen that Intermediate 10 has a smaller total energy than that of Intermediate 9 and so is the more thermodynamically stable isomer. This can be explained Intermediate 10 does has favourable orbital overlap between the π*C=O and the anti-periplaner σCH orbitals stabilising its energy (this is not the case with Intermediate 9).
Intermediate 10 reacts slowly as it is a hyperstable alkene (in other words, the alkene is more stable than its alkane). This is unexpected as Bredt’s rule states that ‘a double bond cannot be placed with one terminus at the bridgehead of a bridged ring system’.[10] However the ring system present in Intermediate 10 is large enough to accommodate the double bond without ‘excessive strain’.[10]
Modelling Using Semi-empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
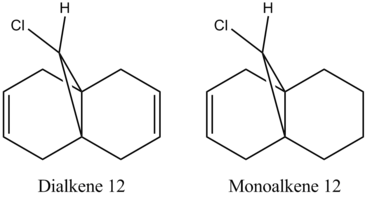
Using ChemBio3D and the MM2 force field function yet again, the energy of Dialkene and Monoalkene 12 was minimised to 17.8975 and 22.3406 kcal/mol repectively. This cleaned up the geometry prior to applying an elctronic method.[11]
Using MOPAC/PM6 MO methods, the geomteries were optimised again and their heat of formation obtained as 19.74062 and -2.43083 kcal/mol for the di- and monoalkene respectively. The further optimisation using the MOPAC/PM6 option.The obtained results are shown to below.
| After MM2 Optimisation | After MOPAC/ PM6 Optimisation | |
|---|---|---|
| Dialkene 12 | 
|

|
| Monoalkene 12 | 
|

|
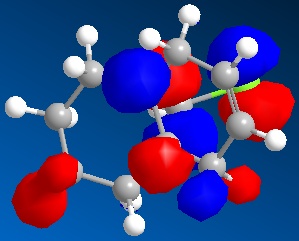
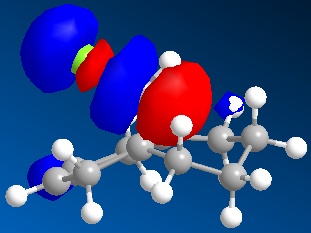
An approximate representation of the valence-electron molecular wavefunctions of both alkenes were also achieved and are shown below.The dialkene has a greater (i.e more positive) heat of formation and therefore is the less stable alkene and more reactive.[6] It has been noted by H. S. Rzepa et al. that Dialkene 12 reacts with reagents that are electrophilic such as dichlorocabene (and per acids) regioselectively ‘on the double bond endo to the chlorine substituent’.,[12] Looking at the molecular orbitals of Dialkene 12, more specifically the HOMO-1, HOMO and HOMO+2, this selectivity can be explained. The LUMO+2 orbital corresponding to the Cl-C π*orbital favourably overlaps with exo π orbital (i.e. HOMO-1) in an antiperiplanar relationship and thus stabilises the later compared to the endo π bond (HOMO) and increases its susceptibility towards electrophilic reagents. Another explanation can be found by looking at the HOMO-1. The electron density is high around the exo π bond and this means that donation can take place between the HOMO-1 and the LUMO+1 and hence weaken the bond between carbon and chlorine. As the Monoalkene 12 does not have an exo π orbital this donation cannot take place, one would expect a stronger carbon-chlorine bond.
| Molecular Orbital | Dialkene 12 | Monoalkene 12 |
|---|---|---|
| HOMO | 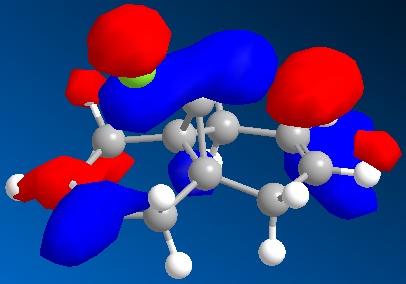
|
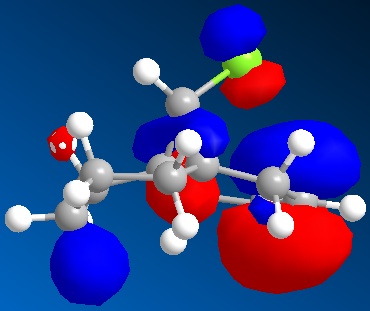
|
| HOMO-1 | 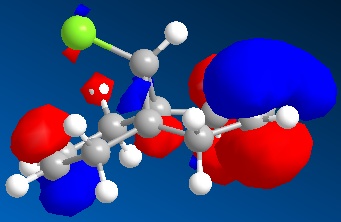
|
|
| LUMO | 
|
|
| LUMO+1 | 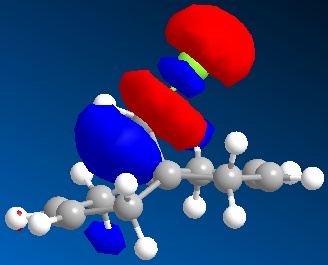
|
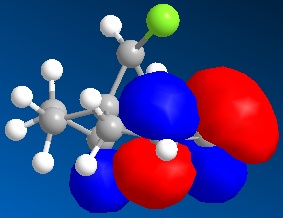
|
| LUMO+2 | 
|

|
Using MOPAC/PM6 optimised geometries of the alkenes and Gaussview 5, The following results for the vibrational frequencies of the C-Cl bond as well as the C=C anti and syn to the chlorine.
| Dialkene 12 Vibrational Frequency/ cm-1 | Monoalkene 12 Vibration Frequency/ cm-1 | |
|---|---|---|
| C-Cl | 770.89 | 774.98 |
| C=C (Anti to Cl) | 1737.04 | 1758.06 |
| C=C (Syn to Cl) | 1757.35 | n/a |
The C-Cl vibrational frequency is 770.89 and 774.98 cm-1 for Dialkene 12 and Monoalkene respectively. This agrees quite well with the value of 780 cm-1 stated by G. Socrates in ‘Infrared and Raman Characterstic Group Frequencies.[13] As the stretching frequency of the monoalkene is greater, it can be said that the C-Cl of the monoalkene is stronger than that of the dialkene as suggested above.

Mini-Project: Direct Amination of 1-Substituted 3,5-Dinitrobenzenes by 1,1,1-Trimethylhydrazinium Iodide
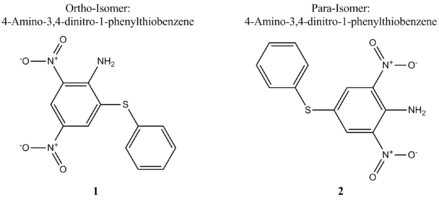
In 2003, while seeking novel methods to use aromatic explosives, A. Shevelev et al. investigated the amination of 1-X-3,5-dinitrobenzenes where X = OMe, OCH2CF3, OCH2CF2CH2H, OPh, SPh or SCH2Ph).,[14] Their report cites the outcome for the amination of each 1-X-3,5-dinitrobenzene with 1,1,1-trimethylhydrazinium (TMHI) using either the base NaOMe or t-BuOH in DMSO. Here, we will focus on the thio-substituted dinitrobenzene (X= SPh).,[14] A. Shevelev and co-workers wanted to expand upon the work of A. Katrizky and others and found that the reaction occurs regioselectively and via the vicarious nucleophilic substitution of hydrogen mechanism (VNS) to give a low yield (23 %) of the ortho and para isomers 1 and 2 in a ratio of 55:45 respectively.[14][15] It is hoped that by using the appropriate modelling techniques, it can be shown why the ortho isomers is the slightly favoured product with regards to its geometry and energy. Molecular modelling allows us to distinguish between the isomers and predict spectroscopic data such as 13C NMR using the GIAO method and IR. This will then be compared to that of the literature where possible.
Isomers 1 and 2 are constitutional isomers. As they differ only in the position of the NH2 group (either ortho or para to the thio-phenyl), one would expect their 13C NMR to differ as the carbons will in slightly different environments and nitrogen is a very electronegative element. One would not expect the IR spectra to vary dramatically.
Why is the Ortho-Isomer Preferred?
The geometries obtained after MM2 and Gaussian optimisation can be seen below:
| Structure After MM2 Optimisation | Structure After Gaussian Optimisation | |
|---|---|---|
| Ortho-Isomer | ||
| Para-Isomer |
Comparison of Energies of the Isomers
It was found by A. Shevelev and co-workers that the ortho-isomer was the preferred product.,[14] This can be rationalised through modelling. Using ChemDrawBio 3D and the MM2 force field option, the total energy of the ortho- and para-isomers was found to be 11.4447 and 18.1870 kcal/mol respectively. From this, we can see that the ortho isomer has a lower total energy compared to the para-isomer and therefore is the more thermodynamically stable. This is touched upon in the report by A. Shevelev et al. but it is only from the calculations performed that this can now be confirmed.,[14]
| Energy Type | Ortho-Isomer | Para-Isomer |
|---|---|---|
| Stretch | 1.6544 | 2.0395 |
| Bend | 7.3046 | 6.0218 |
| Torsion | -11.9319 | -11.9531 |
| Van der Waal's | 16.4209 | 15.4834 |
| Hydrogen Bonding | 7.6807 | 7.8532 |
| Total | 11.4447 | 18.1870 |

From the above result we can see that the o-isomer has a more negative free energy. This means that the forward reaction forming the o-isomer is more spontaneous or favourable than the reaction to produce the p-isomer. From what has been stated already, this is expected and reinforces that the o-isomer is the major product (55:45).
Reaction Mechanism
It must also be noted that if the reaction does indeed proceed via VNS of hydrogen (There is no evidence to suggest otherwise.) it yields another explanation for the ortho-isomer being the major product. The complex formed after the nucleophilic addition to the ortho position can delocalise the negative charge better and therefore is more stable than the corresponding complex after attack at the para-position and hence leads to the major product.,[14]
13C NMR Prediction and Comparison to Literature Values
Here, the chemical shifts obtained for the isomers will be compared to that cited in the literature.[14] Assignments have been made but cannot be compared to the literature as they are not stated. It must also be noted that the 13C spectral data is not complete in the literature without any reasons given. One could assume that there was difficulty interpreting the data as the peaks are quite close together or that the multiplicities ‘hid’ signals.
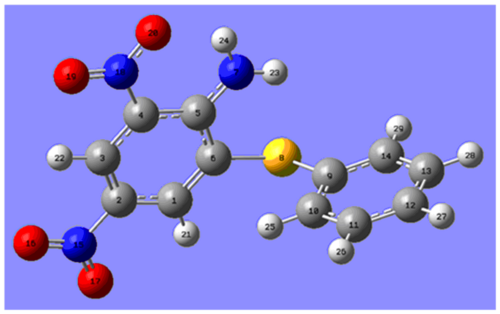

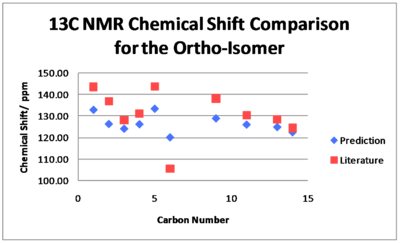
For the ortho-isomer, the chemical shifts obtained tend to agree well with the literature and all the shifts obtained are within 5 ppm of the literature apart from Carbon 6. The predicted shifts are sensical, e.g both Carbon 6 and 9 are attached to the sulphur but as Carbon 6 is closer to the electron donating NH2, it is more shielded and therefore its signal is found at a lower ppm. However, Carbon 6 gives the greatest difference between prediction and experimental. This could be due to Carbon 6 being bonded to a heavier element and therefore a need for Spin-Orbit coupling errors but this is unlikely as the predicted chemical shift for Carbon 9 is in agreement with the literature. The difference between prediction and literature for Carbon 6 could be explained if the geometry of the ortho-isomer was not correct in modelling and so Carbon 6 was in a different environment compared to the actual compound leading to an incorrect prediction. It is also possible however, that a mistake has been made in the literature especially taking in to account that assignments and chemical shifts are missing and the other predicted values concur with the literature. Checking the trace solvents NMR peak list, there is no solvent that would resonate in the region of 105.61 ppm and so if this signal's chemical shift is incorrect in the literature it is due to some other inpurity.[16] Given more time, one would be able to try different conformations of the isomer to see whether the 13C NMR predictions would be closer to the experimental and ascertain whether a mistake had been made.

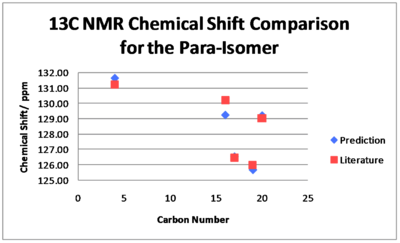
There is greater agreement between the 13C NMR chemical shift predictions and the literature values for the p-isomer. All the predicted chemical shifts are within 0.99 ppm of the values stated in the literature. The degeneracy of Carbon 12 is twice as much as it should be due to the peak overlapping with that of Carbon 16. However, we must note again that chemical shifts are not quoted for each carbon in the literature. It is obvious that the paper of A. Shevelev et al. would have benefitted from the use of molecular modelling in helping with assignments although the multiplicities of the peaks cannot be found this way.
1H NMR Prediction and Comparison to Literature Values
To test the reliability of this technique the proton chemical shifts were also predicted. The achieved results are shown below (Atoms labels remain the same as shown above).



There is a surprising agreement between the predicted 1H NMR chemical shifts and that stated in the literature. Again, no assignments were made in the paper and they failed to identify all the signals. All chemical shifts are within 0.79 ppm of the values cited in the literature (when the mid-point is taken for a range). As seen earlier, the signals of Hydrogen 27 and 26 are so close together the degeneracy is twice for the former than expected. The literature seems to have stated incorrect multiplicities. Although the calculations cannot give any information on this aspect of 1H NMR it is quite clear (assuming geometry and assignments are correct) that Hydrogens 22 and 24 are in different environments and therefore cannot correspond to a broad singlet integrating to two hydrogens but two separate signals corresponding to two different hydrogens as suggested by the calculations performed. As this peak is described as a broad singlet it is possible that this error is due to the resolution of the spectrometer. Poor resolution would cause the signals to not be well resolved and therefore cause confusion. This however is not an issue with the technique used. This explanation can also be used to rationalise how A. Shevelev et al. suggested a singlet for Hydrogens 21 and 29 which again clearly are different (but slightly similar environment). No information is provided about the spectrometer used so this idea cannot be confirmed. It also seems that they have taken the easy way out and have just described peaks in the region as a multiplet, rather than identifying each signal. As noted above, the peaks in the spectrum may overlap resulting in difficulty intepretating it but the use of programs such as MestreNova and the calculations performed show that complete assignment is possible.

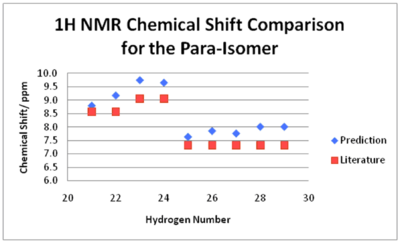
The 1H NMR prediction for the para-isomer is also in agreement with the literature (when a mid-point is taken for any ranges stated). All predicted chemical shifts are within 0.70 ppm of the literature. Again, there is the issue of assigning with the multiplicities. For example, literature suggests a doublet for Hydrogens 13 and 24, whereas the calculations performed show that they should correspond to two different peaks. As there is free rotation around the C-N bond of the NH2 group, it is possible that the spectrum obtained by A. Shevelev and co-workers showed a time-averaged signal. The calculations performed consider the isomer as static and can therefore distinguish between the two hydrogens and both signals are seen. This could be confirmed if A. Shevelev et al. re-ran their spectrum at a lower temperature. At a lower temperature, rotation would be slower and resolved peaks for both hydrogens would be achieved. This is also the case for Hydrogens 21 and 22. Again, A. Shevelev and co-workers have simply assigned a multiplet to five different signals.
3J H-H Prediction
The literature only states one 4J coupling constant and no 3J coupling constants. However, the 3J couplings were predicted using the program Janocchio. This program can only calculate coupling constants 3 bonds apart and so it was not possible to calculate the 4J coupling contants and compare to the literature. Using the aforementioned program, the angle between the hydrogens three bonds apart were also noted. It was shown that the hydrogens are not actually co-planar, as expected for an aromatic ring but deviate very slightly from co-planarity by 0.1 – 0.5⁰. The coupling constants obtained make sense as it is known that ortho coupling of aromatic protons should be in the region of 8Hz. There is very little difference in coupling constants between the isomers. Only four of the eight possible coupling constants are quoted as for example, 3JH25-H26 equals 3JH26-H25.

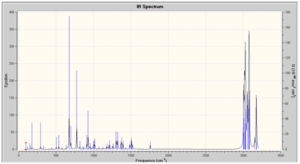
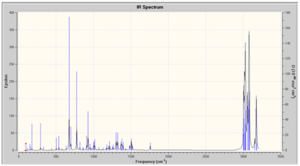
IR Spectra Prediction
The IR spectra was predicted, however as values are not stated in the literature it is not possible to compare. A second source of data was looked for but without success. Vibrations have been assigned below.



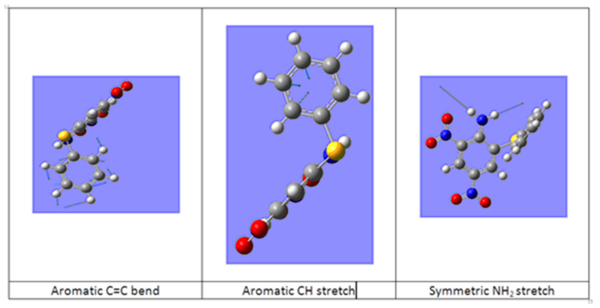
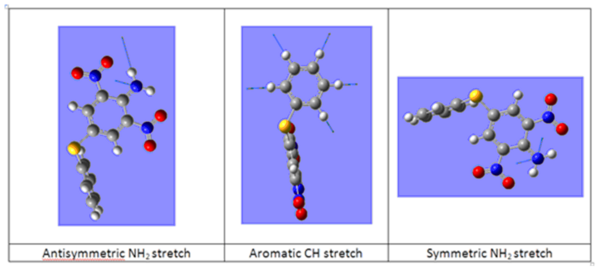
In both spectra, there are two bands found at greater than 3500 cm-1, these correspond to the symmetric and antisymmetric stretch of the NH2 group. From their position and that there are two peaks we know that the isomers the NH2 substituent is a primary amine bonded to an aromatic functionality. There are two aromatic rings present in the isomers and this is confirmed by the peaks at 1633.71 and 3214.69 cm-1 for the o-isomer and 1632.07 and 3215.14 cm-1 for the p-isomer correspond to the aromatic C=C bend and CH stretch. These peaks are weak in both spectra but obviously so more in the p-isomer due to greater symmetry. The NO2 stretches are characteristically strong in both spectra.[17]
Conclusions
The above work has highlighted how molecular modelling can be helpful to chemists. Throughout the exercises it has been shown that such methods enable us to analyse reactions easily without having to step into a laboratory! The mini-project went on to show the effectiveness of molecular modelling when it comes to trying to critically evaluate literature and distinguishing between here. It was even noted that the literature would have benefitted from using molecular modelling prior to publication in order to improve the report. The techniques used here to predict NMR spectra were in good agreement with the literature. However, a let-down of this mini-project was not being able to compare the predicted IR spectra with any literature values. The effectiveness of the modelling in that instance cannot therefore be commented on.
References
- ↑ 1.0 1.1 https://www.ch.ic.ac.uk/wiki/index.php/Mod:organic
- ↑ https://www.ch.ic.ac.uk/wiki/index.php/Mod:molecular_mechanics
- ↑ http://www.ch.ic.ac.uk/motm/porphyrins/introDA.html
- ↑ 4.0 4.1 Mechanistic Aspects of Diels-Alder Reactions: A Critical Survey:DOI:10.1002/anie.198007791
- ↑ Clayden, J. et al, Organic Chemistry, OUP Oxford, 1st Edn., 2001, pp.916
- ↑ 6.0 6.1 6.2 Carey, F. A. et al, Advanced Organic Chemistry: Sructure and Mechanisms, Springer, 4th Edn., 2000, pp.123 Cite error: Invalid
<ref>tag; name "Adv. Org. Chem." defined multiple times with different content - ↑ 7.0 7.1 7.2 7.3 Shultz, A. G., et al., J. Org. Chem, 1986, 51, 838DOI:10.1021/jo00356a016
- ↑ Paquette, L. et al., Tetrahedron Letters, 1991, 319; :DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ Mislow, K., Introduction to Stereochemistry, Dover Publications, 1st Edn., 2003, pp.78
- ↑ 10.0 10.1 www.iupac.org/goldbook/B00732.pdf
- ↑ https://www.ch.ic.ac.uk/wiki/index.php/Mod:organic
- ↑ Rzepa, H. S. et al., J. Chem. Soc., Perkin Trans 2, 1992, 447; DOI:10.1039/P29920000447
- ↑ Socrates, G., Infrared and Raman Characteristic Group Frequencies, WileyBlackwell, 3rd Edn., 2001, pp.62
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Shevelev, A. G et al., J. Org. Chem., 2003, 68, 2498:DOI:10.1002/chin.200330104 Cite error: Invalid
<ref>tag; name "Mini-Project Lit." defined multiple times with different content - ↑ Katrizky, A. et al., J. Org. Chem.,, 1986, 21, 5040: DOI:10.1021/jo00375a062
- ↑ Kotlyar, V et al, J. Org. Chem., 1997, 62, 7512:DOI:10.1021/jo971176v
- ↑ Smith, B. C., Infrared spectral interpretation: a systematic approach , CRC Pressl, 1st Edn., 1998