Rep:Mod:Computationalreport1C
Synthesis and Computational Lab: 1C
Part 1: Introduction to Molecular Mechanics
Introduction to MMFF94 and MMFF94s
With the improvement of computational data and computer processing power, the usage of molecular mechanics/dynamics forcefield has become very integrated in predicting properties of chemical molecules. A molecular mechanics/dynamics forcefield should be able to predict characteristics such as molecular geometries, conformational/stereoisomeric energies, vibrational frequencies etc. to a reasonable accuracy as compared to experimental-derived values.
Building on work from Kollman et al. s’ AMBER forcefield and Allinger et al. s’ MM1, MM2, MM3 and MM4, Merck Research Laboratories have managed to optimized and improved the previous computational algorithms and produced MMFF94 (Merck Molecular Force Field 94). One of the most prominent advantages of MMFF94 over other preceeding work is its enhanced prediction accuracy. The reason lies in the fact that MMFF94’s core parameterization utilizes an unprecedently large amount of computational data obtained from ab initio calculations1.
Subsequently after the development of MMFF94, a new “static” variant of the MMFF was introduced i.e. the MMFF94s. Unlike MMFF94 that was developed for the use of molecular dynamics simulations (which can be expensive), MMFF94s was developed with the purpose of energy minimization studies in mind. Although both systems provide identical results for most systems, they differ for amides and unsaturated amines which contain resonance-delocalized trigonal nitrogen atoms2.
In this computational exercise, MMFF94s is used predominantly as oppose to the alternative Universal Force Field (UFF). Although versatile across the entire periodic table, UFF is derived from simple rules and atomic parameters such as fixed hybridization that increases the likelihood of erroneous structural and energetic predictions3.
Forms of MMFF94s
Steric energy of a molecule is the energy arise due to its geometry or conformation. Energy minimization of steric energy process ensures that the molecule is in its lowest energy conformation.
MMFF94s assumes that the total steric energy of a molecule can be found by the sum of a few specific interactions in a molecule.
ES = EBS + EAB + E SB + ET + EOOP + EVdW + EEE
where the bonded interactions are:
ES = Total Steric energy
EBS = Total Bond Stretching energy
EAB = Total Angle Bending energy
ESB = Total Stretch Bending energy
ET = Total Torsional Energy
EOOP = Total Out-Of-Plane Bending energy, and
where the non-bonded interactions are:
EVdW = Total Van der Waals Energy
EEE = Total Electrostatic Energy
| Types of Energies | Explanation |
|---|---|
| Bonded Interactions | |
| Total Bond Stretching Energy | The total bond stretching energy is related to the vibration energy of the molecules in comparison to the equilibrium bond length. Whenever a bond deviates from the equilibrium bond length, the energy value increases.
MMFF94s uses a quartic function that has the force constant term (relate to bond strength). The quartic function term is superior to the cubic function term (used in MM2) in the sense that it avoid the “cubic stretch catastrophe” where progressive elongation of molecule bond length leads to negative infinity. |
| Total Angle Bending Energy | Similar to Total Bond Stretching Energy, the total angle bending energy is also related to the vibration energy of the molecules about the equilibrium bond length. However, unlike EBS that measures the bond strength, EAB is associated with the stiffness of the molecules based on the Hookiean potential. Whenever bond angles deviate from the norm during vibration, this energy term increases as well. |
| Total Stretch-Bending Energy | This energy term takes into account of the energy due to increase in bond length when bond angle between two atoms changes. |
| Total Torsional Energy | Total torsional energy is the energy required for intermolecular rotation about the bonds. In molecule mechanics, however, this term is often used as a correction that must be added or subtracted to the total energy of the molecule to fit a quantum mechanical calculation about the dihedral angles of bonds. |
| Total Out-Of-Plane Bending Energy | This energy term refers to the energy needed to deform the dihedral angle (zero degrees) of a planar group of atoms such as carbonyl or amide functional groups. |
| Non-Bonded Interactions | |
| Total Van der Waals Energy | The total Van der Waals energy term examines the intramolecular interactions such as repulsion or attraction between non-bonded atoms in the molecule. This term is crucial in determining the preferred conformation of the molecule.
MMFF94s employs the novel “Buffered-14-7”. The name is derived from a buffering constant and the 14th and 7th power dependencies of the repulsive and attractive energy in the equation. |
| Total Electrostatic Energy | The total electrostatic energy functions take into account of the Coulombic potential and partial charge/dipole/atomic point charges interactions within the molecule. |
Part 2 : Conformational Analysis Using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
Endo and exo adduct from the Dimerization of Cyclopentadiene
Cyclopentadiene will undergo dimerization by Diels-alder reaction to form either an endo or exo adduct.
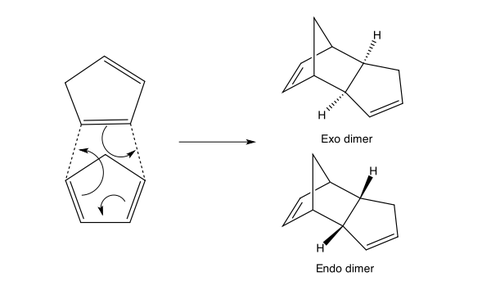
Stereoselectivity of Dimerization of Cyclopentadiene
Although the exo adduct is thermodynamically more stable than the endo adduct, the endo adduct is form preferentially. Using the QST2 method, the transition state of the Diels Alder dimerization was found. It was discovered that due to the secondary orbital interactions in the endo transition state, the endo dimer is formed preferentially (the kinetic product). Hence, it is proven that the Diels Alder dimerization between two cyclopentadiene are kinetically controlled.
Asynchronous Bond Formation of Dimerization of Cyclopentadiene
The initial study of the molecular orbitals of the transition states seemed a bit peculiar. Instead of getting a LUMO with symmetrical orbitals, the calculations showed a HOMO that is asymetric at the carbons where the new sigma bonds will be formed. Running an IRC further, it soon became clear that the asymmetry of the HOMO leads to an asychronous bond formation before leading to the product.
| IRC plot following the Endo Transition State | IRC following the Endo Transition State (click to animate) | Asymmetry of orbitals at the carbons where the new sigma bonds will be formed |
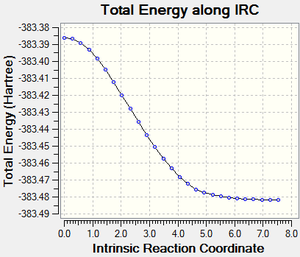 |
 |
 |
Comparison of Energies between the Endo and Exo adduct of Dicyclopentadiene
| Endo Adduct of Dicyclopentadiene dimerization | Exo Adduct of Dicyclopentadiene dimerization | ||||||
|
|
Using MMFF94s ab initio calculations, the energies for the exo dimer and the endo dimer are derived.
| Energies | ||||||||
| ES | EBS | EAB | ESB | ET | EOOP | EVdW | EEE | |
| Exodimer | 55.374 | 3.543 | 30.772 | -2.041 | -2.728 | 0.015 | 12.800 | 13.014 |
| Endodimer | 58.191 | 3.468 | 33.187 | -2.082 | -2.950 | 0.023 | 12.360 | 14.186 |
The MMFF94s steric energy (after minimization) for the exo dimer is lower than that of the endo dimer. This is reflected by the large difference for the EEE between the endo dimer and the exo dimer due to unfavourable steric repulsion between the two “arched down” cyclopentene rings.
Hydrogenation of Dicyclopentadiene
Hydrogenation of the endo dicyclopentadiene (DCPD) would lead to two potential products: dihydro product 1 and dihydro product 2.

Using MMFF94s, the energy of the two dihydro products are calculated.
| Energies | ||||||||
| ES | EBS | EAB | ESB | ET | EOOP | EVdW | EEE | |
| Dihydro Product 1 | 50.123 | 3.308 | 30.864 | -1.927 | 0.060 | 0.015(2) | 13.281 | 5.121 |
| Dihydro Product 2 | 41.257 | 2.823 | 24.685 | -1.657 | -0.379 | 0.000(2) | 10.639 | 5.147 |
| Tetrahydro Product | 39.042 | 2.730 | 25.151 | -1.676 | 1.287 | 0.000 | 11.551 | 0.000 |
From the MMFF94s calculation, it can be seen that dihydro product 2 has a lower steric energy than dihydro product 1. Therefore, dihydro product 2 is the thermodynamic product for the hydrogenation of dicylopentadiene. And indeed, this was what was observed by Skala et al.. He observed that the first hydrogenation occur at the norborene part of the molecule (rate of hydrogenation several times faster than that in the cyclopentene ring)6. The first heat of hydrogenation at the norborene is 138.8 kJ/mol while the second hydrogenation at the cyclopentene right is 109.5 kJ/mol6.
From the above results, it is also obvious that tetrahydro product is much more stable than either dihydro product. And yet, studies have shown that the tetrahydro product will only form after prolonged hydrogenation under high temperature. However, subjecting DCPD to high temperature leads to low yield due to cracking of DCPD.
Since thermodynamically it is predicted to form favourably, the fact that it does not could only mean that the hydrogenation of DCPD to tetrahydrodicyclopentadiene (TH-DCPD) is kinetically controlled. Most possibly, the transition state of the hydrogenation of DCPD is highly unstable hence it is unlikely to form.
Atropisomerism is a type of stereoisomerism that is form as a result of hindered rotation about a single bond.
In the case of the 6,6’-dinitro-2,2’-diphenic acid, rotation about the bonds joining the two substituted phenyl rings would have to overcome large steric interaction between the bulky substituents. The barrier of rotation is high enough for the two conformers to be isolated individually.
In the synthesis of taxol, one of the key intermediates exhibit atropisomerism.
Atropisomerism in Taxol Synthesis
For the key intermediate, rotation about the carbon-carbon bonds would lead to two types of atropisomers, each with either the carbonyl group poining upwards or downwards in relative to the bridge.

Once again by using MMFF94s ab initio calculations, the steric energies for the two intermediates are illustrated as below:
| Energies | ||||||||
| ES | EBS | EAB | ESB | ET | EOOP | EVdW | EEE | |
| Intermediate 9 | 70.571 | 7.743 | 28.356 | -0.062 | 0.004 | 0.949 | 33.293 | 0.288 |
| Intermediate 10 | 60.553 | 7.599 | 18.838 | -0.139 | 0.199 | 0.844 | 33.269 | -0.056 |
Analyzing the data above, the largest energy difference between Intermediate 9 and Intermediate 10 stems from the EAB which is related to the strain of the ring. Intermediate 9 has an excess of 9.518 kcal/mol EAB as compared to Intermediate 10. This comes as a surprise as intermediate 9 is predicted to be more stable alkene.
The rationale behind the prediction of the stability of intermediate 9 stems from the fact that the through-space distance of the upward-pointing carbonyl oxygen with the carbon-carbon is further than the through space distance between the downward-pointing carbonyl oxygen with the -CH2-CH2- fragment at the norbornene part in intermediate 10.
Hyperstable Olefins
Intermediate 9 and Intermediate 10 does not react readily with other reagents during functionalization. This is due to the hyperstability effect found in hyperstable olefins.
Hyperstable olefins are alkenes that contain less strain in the ring (i.e. negative olefin strain values) compared to the parent hydrocarbon. To study this further, further MMFF94s calculation was done to compare Intermediate 10 with the parent hydrocarbon.

The energies that we calculated are tabulated as shown below:
| Energies | ||||||||
| ES | EBS | EAB | ESB | ET | EOOP | EVdW | EEE | |
| Intermediate 10 | 44.878 | 5.211 | 15.488 | -0.267 | -1.655 | 0.998 | 25.014 | 0.088 |
| Parent Hydrocarbon | 50.564 | 4.692 | 21.979 | 0.109 | -0.958 | 0.247 | 24.495 | 0.000 |
Scrutinizing the results, the largest energy difference between intermediate 10 and the parent hydrocarbon stems from the Total Angle Bending Energy, EAB. Since the Total Angle bending energy is related to the angle strain in the molecules, it is apparent that the parent hydrocarbon has more ring strain as compared to the bridgehead olefin.
Part 3 : Spectroscopic Simulation Using Quantum Mechanics
Spectroscopy of an Intermediate Related to the Synthesis of Taxol
Molecule 17 and 18 are derivatives of intermediate 9 and 10. In this exercise, MMFF94s is used to predict the 1H and 13C NMR of molecule 17 and molecule 18.

NMR Spectra of Molecule 17
Endodimer |
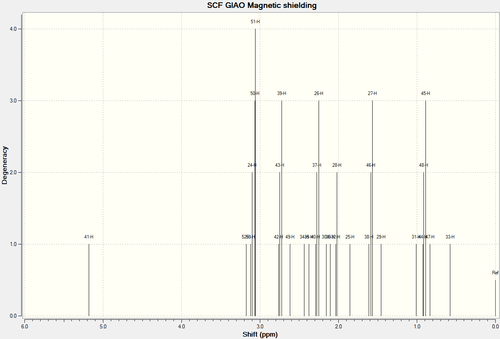
1H NMR Spectra of Molecule 17
| 1H NMR Spectra of Molecule 17 Full Spectra | 1H NMR Spectra of Molecule 17 Truncated Spectra |
 |
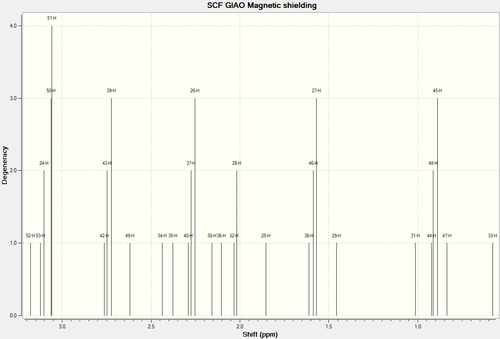 |
| H-Atoms | Chemical Shift (ppm) | Integration Height/Degeneracy | Literature values | Comments |
| 41 | 5.1825 | 1 | 5.21 | The first three results were fairly straight forward to be deduced and correlate wit experimental data.
2. Some of the peaks in the literature remains unresolved and are given as multiplets or grouped together. |
| 52 | 3.1775 | 1 | 2.99 | |
| 53,52,50,51 | 3.0867 | 4 | 3.40-3.10 | |
| 42,43,39 | 2.7445 | 3 | ||
| 49 | 2.6187 | 1 | ||
| 34 | 2.4371 | 1 | ||
| 35 | 2.3782 | 1 | ||
| 40,37,26 | 2.2730 | 3 | ||
| 30 | 2.1566 | 1 | ||
| 36 | 2.1033 | 1 | ||
| 32,28 | 2.0259 | 2 | ||
| 25 | 1.8535 | 2 | ||
| 38,46,27 | 1.5898 | 3 | 1.25 | |
| 29 | 1.4557 | 1 | ||
| 31 | 1.0123 | 1 | ||
| 44,48,45 | 0.9087 | 3 | 1.10 | |
| 47 | 0.8354 | 1 | ||
| 33 | 0.5790 | 1 |
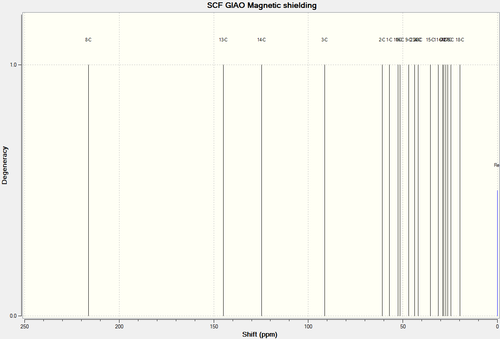
13C NMR Spectra of Molecule 17
| 13C NMR Spectra of Molecule 17 Full Spectra | 13C NMR Spectra of Molecule 17 Truncated Spectra |
 |
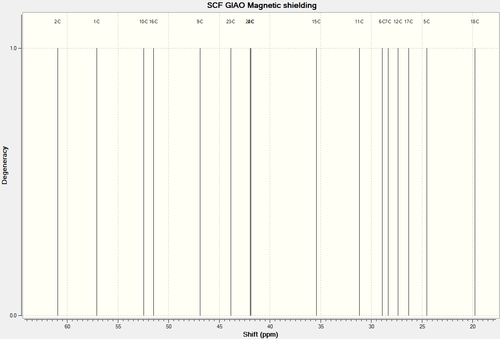 |
| C-Atoms | Chemical Shift (ppm) | Integration Height/Degeneracy | Literature Values | Image of carbon skeleton |
| 8 | 216.2725 | 1 | 218.79 |  |
| 13 | 144.8626 | 1 | 144.63 | |
| 14 | 124.7193 | 1 | 125.33 | |
| 3 | 91.3340 | 1 | 72.88 | |
| 2 | 60.9568 | 1 | 56.19 | |
| 1 | 57.0811 | 1 | 52.52 | |
| 10 | 52.4663 | 1 | 48.50 | |
| 16 | 51.4857 | 1 | 46.80 | |
| 9 | 46.9196 | 1 | 45.76 | |
| 23 | 43.8647 | 1 | 39.80 | |
| 22 | 41.9450 | 1 | 38.81 | Comments |
| 4 | 41.8724 | 1 | 35.85 | The carbon bonded to the oxygen atom is the most deshielded, as is shown in the predicted values and the experimental values. There is a deviation between the values. Quite possibly, this is due to the fact that in reality, the carbonyl functional group is pointing slightly towards the equatorial position rather than a lateral position relative to the ring (as is in the input for Gaussian calculation)
|
| 15 | 35.4348 | 1 | 32.66 | |
| 11 | 31.1964 | 1 | 28.79 | |
| 6 | 28.9432 | 1 | 28.29 | |
| 7 | 28.3640 | 1 | 26.88 | |
| 12 | 27.3767 | 1 | 25.66 | |
| 17 | 26.3311 | 1 | 23.86 | |
| 15 | 24.5483 | 1 | 20.96 | |
| 18 | 19.7788 | 1 | 18.71 |
NMR Spectra of Molecule 18
Endodimer |
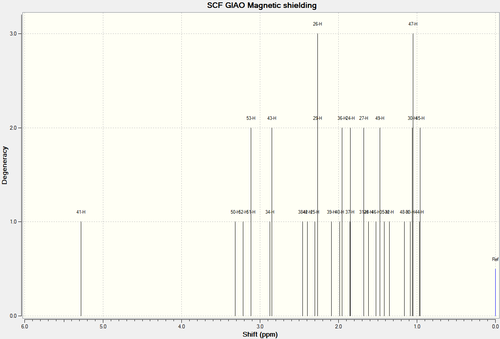
1H NMR Spectra of Molecule 18
| 1H NMR Spectra of Molecule 17 Full Spectra | 1H NMR Spectra of Molecule 17 Truncated Spectra |
 |
 |
| H-Atoms | Chemical Shift (ppm) | Integration Height/Degeneracy | Literature values |
| 41 | 5.2832 | 1 | 5.21 |
| 50 | 3.3175 | 1 | 3.00-2.70 |
| 52 | 3.2153 | 1 | |
| 51,53 | 3.1161 | 2 | |
| 34,43 | 2.8606 | 2 | |
| 38 | 2.4549 | 1 | |
| 42 | 2.3968 | 1 | |
| 25,29,26 | 2.2796 | 3 | |
| 39 | 2.0844 | 1 | |
| 40,36 | 1.9699 | 2 | |
| 37,24 | 1.8519 | 2 | |
| 31,27 | 1.6780 | 2 | |
| 28 | 1.6175 | 1 | |
| 46,49 | 1.4979 | 2 | |
| 35 | 1.4150 | 1 | |
| 32 | 1.3531 | 1 | |
| 48 | 1.1606 | 1 | |
| 33,30,47 | 1.0653 | 3 | 1.03 |
| 44,45 | 0.9643 | 2 |
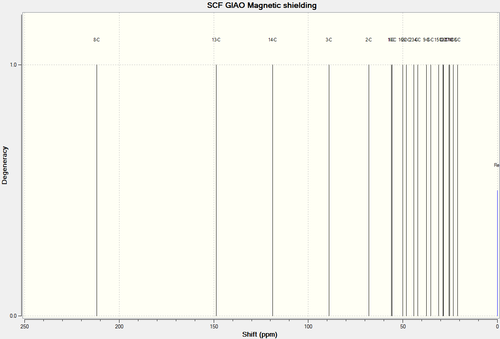
13C NMR Spectra of Molecule 18
| 13C NMR Spectra of Molecule 18 Full Spectra | 13C NMR Spectra of Molecule 18 Truncated Spectra |
 |
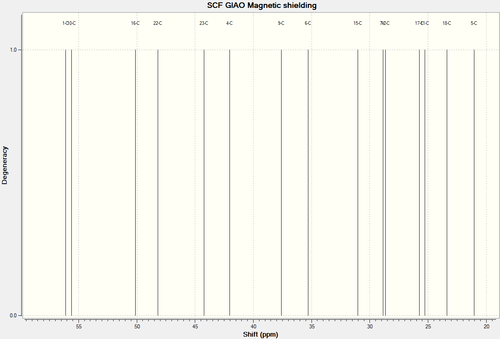 |
| C-Atoms | Chemical Shift (ppm) | Integration Height/Degeneracy | Literature Values |
| 8 | 211.8325 | 1 | 211.49 |
| 13 | 148.6673 | 1 | 148.72 |
| 14 | 118.8373 | 1 | 120.90 |
| 3 | 89.0869 | 1 | 74.61 |
| 2 | 67.8886 | 1 | 60.53 |
| 1 | 56.1133 | 1 | 51.30 |
| 10 | 55.5998 | 1 | 50.94 |
| 16 | 50.1124 | 1 | 45.53 |
| 22 | 48.2030 | 1 | 43.28 |
| 23 | 44.2222 | 1 | 40.82 |
| 4 | 42.0354 | 1 | 38.73 |
| 9 | 37.5865 | 1 | 36.78 |
| 6 | 35.3106 | 1 | 35.47 |
| 15 | 31.0459 | 1 | 30.84 |
| 7 | 28.8476 | 1 | 30.00 |
| 12 | 28.6623 | 1 | 25.56 |
| 17 | 25.7393 | 1 | 25.35 |
| 11 | 25.2603 | 1 | 22.21 |
| 18 | 23.3944 | 1 | 21.39 |
| 5 | 21.0478 | 1 | 19.83 |
Part 4 : Analysis of the Properties of the Synthesized Alkene Epoxides
The two catalytics systems
Assymmetric epoxidation of prochiral alkenes may lead to product with different stereochemical outcome as illustrated as below. To solve this problem, different types of asymmetric epoxidation catalyst have been synthesized. In this study, we shall be focusing on Shi asymmetric fructose catalyst as well as Jacobsen asymmetric catalyst.

The Shi Asymmetric Fructose Catalyst
Molecule 21 is the stable precursor of molecule 22, the active catalyst. Molecule 21 can be converted to molecule 22 by using either persulfuric acid or potassium peroxymonosulphate.
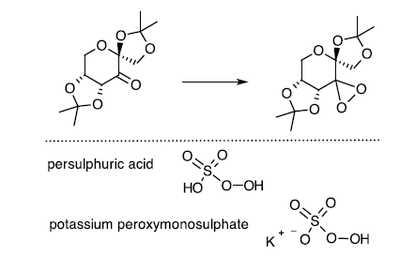
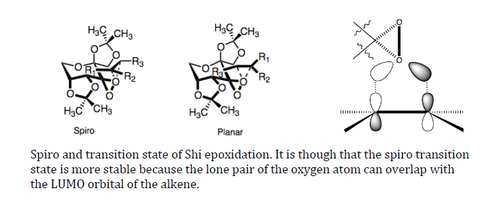
The transition state of the Shi epoxidation was a subject of debate for a few moments. However, recent studies have led to the conclusion that the spiro transition state is more favored than the planar transition state. The spiro transition state is the transition that has its dioxirane perpendicularly bisecting the alkene double bond. This is thought to be more favourable due to interactions between the lone pairs of the oxygen with the LUMO of the alkene.
The Jacobsen Asymmetric Catalyst
Molecule 23 is the stable precursor of molecule 24, the active catalyst. Molecule 23 can be converted to molecule 24 by using sodium perchlorate.
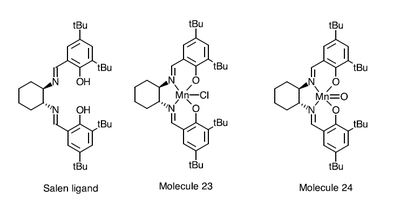
Jacobsen catalyst is biphasic. The first phase involves the transfer of oxygen to molecule 23. Sodium perchlorate oxidizes Mn(III) to Mn(V).
In the second step, Jacobsen catalyst acts via a radical mechanism. It is thought to be so because epoxidation of trans-alkene by Jacobsen catalyst yield cis-alkene as well. A radical mechanism will lead to the lost of stereochemical information of the molecule.
Crystal structure of the two catalyst
Molecule 21
| Analysis of Crystal Structure of Molecule 21 | ||||||||||||
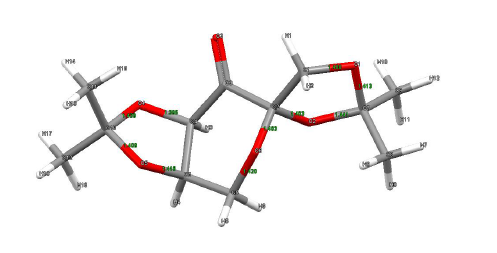 | ||||||||||||
| Bond | Bond Lenghts (Angstrom) | Evaluation | ||||||||||
| O1-C1 | 1.400 | 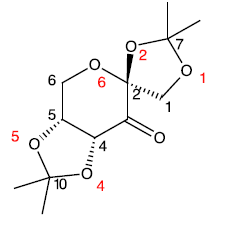 The normal bond length of an C-O bond is 1.430 angstrom. The most interesting aspect here is O4 and O2. The lone pair of O4 is antiperplanar to the sigma* orbital of the carbonyl group while the lone pair of O2 is anti periplanar to the carbonyl pi* orbital. Donation of electron can occur because of the correct symmetry. Due to the mixing of the orbitals, the C2-O2 and C4-O4 has been conferred with a certain degree of double bond character. However, due to the lone pair on oxygen being now donated to the carbonyl antibonding orbitals, the O2 and O4 is slightly “electron deficient” and electron density will be pulled away from O2 and O4. That would explain why the bond length increase for O2-C7 and O4-C10. Increase in bond length is an indicator of the weakening of the bond. | ||||||||||
| O1-C7 | 1.413 | |||||||||||
| O2-C7 | 1.441 | |||||||||||
| O2-C2 | 1.403 | |||||||||||
| O4-C4 | 1.396 | |||||||||||
| O4-C10 | 1.439 | |||||||||||
| O5-C10 | 1.409 | |||||||||||
| O5-C5 | 1.416 | |||||||||||
| O6-C2 | 1.403 | |||||||||||
| O6-C6 | 1.420 | |||||||||||
Molecule 23
| Analysis of Crystal Structure of Molecule 23 | ||||||||||||
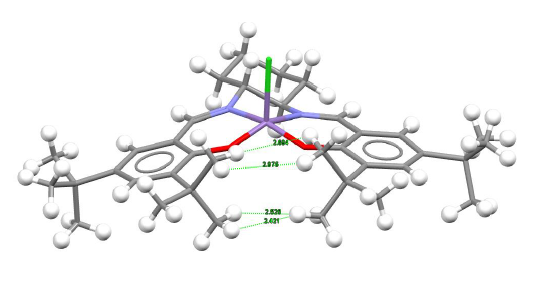 | ||||||||||||
| The most outstanding feature from this molecule is the fact that is adopt a puckered form of the square base pyramidal form. This is no surprise that the molecule is almost planar due to the electron delocalization around each side of the salen ligand.
The tery-butyl hydrogen are oriented closely to one another. Non-covalent interaction studies indicated that repulsion would occur for H---H bond length that are less than 2.1 angstrom while favourable interaction may be dominant with bond length above 2.4 angstrom. It was discovered that all 4 hydrogens have bond length more than 2.4 angstrom hence there should not be any significant repulsion between them. Instead, certain amount of attractive force will ensue and that contributes to the overall stability of the molecule | ||||||||||||
The calculated NMR properties of the product
The two products that have been chosen are styrene and trans-stilbene.
| (R)-Styrene | (S)-Styrene | |
| 1H NMR data |
Shift (ppm) Degeneracy
7.4891704929 4.0000
7.2988172444 1.0000
3.6627082199 1.0000
3.1170156088 1.0000
2.5382383043 1.0000
|
Shift (ppm) Degeneracy
7.4892967873 4.0000
7.2987138125 1.0000
3.6632689921 1.0000
3.1151709109 1.0000
2.5339209685 1.0000
|
| 1H NMR Spectra |  |
 |
| 13C NMR data |
Shift (ppm) Degeneracy
135.1098747604 1.0000
124.1186533632 1.0000
123.4150641684 1.0000
122.9584694331 2.0000
118.2653162569 1.0000
54.0265785300 1.0000
53.4853575062 1.0000
|
Shift (ppm) Degeneracy
135.1323742030 1.0000
124.1343038829 1.0000
123.4137039868 1.0000
122.9558208084 2.0000
118.2690258882 1.0000
54.0599580762 1.0000
53.4684920923 1.0000
|
| 13C NMR Spectra |  |
 |
| (R,R)-Trans-stilbene | (S,S)-Trans-stilbene | |
| 1H NMR data | Shift (ppm) Degeneracy
7.5705724829 2.0000
7.4791732656 8.0000
3.5390102503 2.0000
|
Shift (ppm) Degeneracy
7.5705651164 2.0000
7.4791779628 8.0000
3.5374502607 2.0000
|
| 1H NMR Spectra |  |
 |
| 13C NMR data | Shift (ppm) Degeneracy
134.0942417727 2.0000
124.2206838184 2.0000
123.5192390024 2.0000
123.2125901307 2.0000
123.0785800542 2.0000
118.2583039589 2.0000
66.4212770637 2.0000
|
Shift (ppm) Degeneracy
134.0899175142 2.0000
124.2224499528 2.0000
123.5180152862 2.0000
123.2113538650 2.0000
123.0745448885 2.0000
118.2635789492 2.0000
66.4249347217 2.0000
|
| 13C NMR Spectra |  |
 |
Assigning the absolute configuration of the product
The two products that have been chosen are styrene and trans-stilbene.
| (R)-Styrene | (S)-Styrene | (R,R)-Trans-stilbene | (S,S)-Trans-stilbene | |
| Predicted ORP | -24 deg | 32.1 deg | 296 deg | -205.2 deg |
| Experimental ORP | -24 deg | 32.1 deg | 296 deg | -205.2 deg |
| Experimental conditions | 1.00g/100ml, chloroform, 21 degree Celcisus, 589 nm wavelength | 1.02g/100ml (99% ee), chloroform, 25 degree Celsius, 589 nm wavelength | 1.01g/100ml, ethanol, 22 degree Celsius, 589 nm wavelength | 0.6g/100ml, ethanol, 20 degree Celsius, 589 nm wavelength |
| Predicted VCD Spectrum |  |
 |
 |
 |
All the assignments given in the literature reports are correct although the predicted value differs slightly with the experimental values
Analysis of the Transition State for Reactions
Predicting Enantiomeric Excess based on Transition State energies
One of the energy output from Gaussian calculation is the “sum of electronic and thermal free energies”. This is Gibbs Free Energy at 298.15K and 1 atm.
Using this term and invoking a variant of the Curtin-Hammett Principle, it is possible to calculate a predicted enantiomeric excess of one enantiomer over the other.
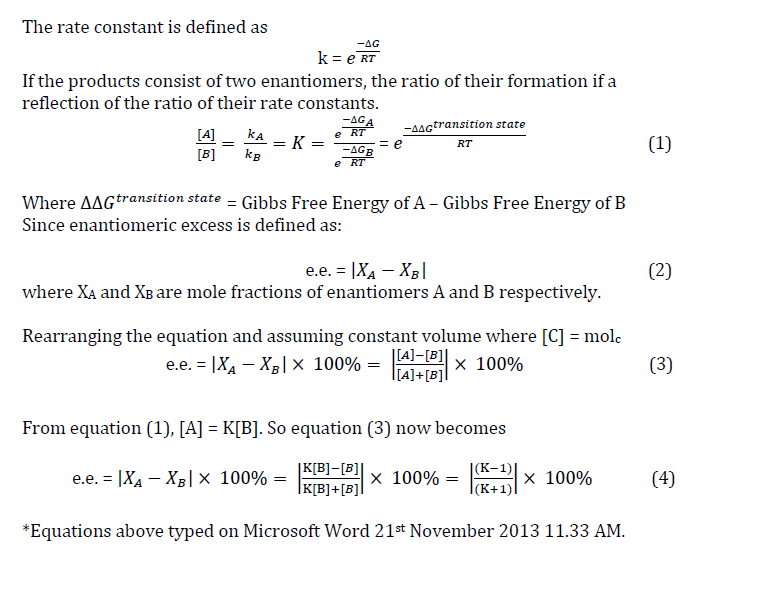
Using the derivations from above and equation (4), we analyze the enantiomeric excess of beta-methyl styrene and the results are tabulated as below:
| Free Energy of (R,R) transition state | Free Energy of (S,S) transition state | Difference in free energies (Hartrees) | K | Enantiomeric Excess (%) |
| -1343.022970 | -1343.017942 | -0.005028 | 4.88E-03 | 99.03 |
| -1343.019233 | -1343.015603 | -0.003630 | 2.14E-02 | 95.80 |
| -1343.029272 | -1343.023766 | -0.005506 | 2.94E-03 | 99.41 |
| -1343.032443 | -1343.024742 | -0.007701 | 2.88E-04 | 99.94 |
The above hartree units are converted to J/mol for calculation of K where 1 hartress = 4.3597E-18
Studying all the free energies of the (R,R) series and compare them with the (S,S) series, it comes as no surprise that (R,R) product is favoured over (S,S) product as (R,R) is more stable. Indeed, this corresponds with the literature value reporting 92% ee of (R,R) products over that of (S,S).
Analysis of the Transition State of Epoxidation
Shi Fructose Catalyst
For this section, I will be analyzing the the transition state of 1) styrene and 2) trans-stilbene with the Shi fructose catalyst.
(R) and (S)-styrene transition state
The transition state for the formation of (R)-styrene oxide and (S)-styrene oxide are given as below:
| (R)-styrene oxide | (S)-styrene oxide | |||||||
| Structure of Catalyst |
|
| ||||||
| Approach of ligand |  |
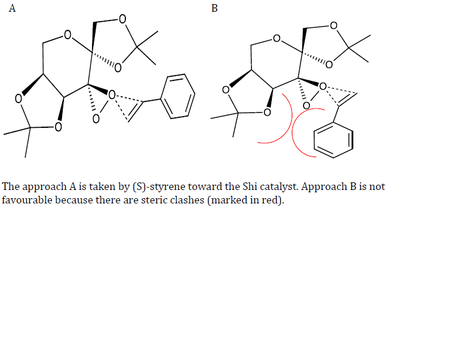 |
In the transition state for (R)-styrene, it was discovered that the Re face is reacting with the oxygen of the dioxirane. The phenyl ring is oriented endo with respect to the fructose catalyst. However, the (S)-styrene is reacting at the Si face and the phenyl ring is oriented exo relative to the catalyst.
The approach taken by (R)-styrene and (S)-styrene are restricted to only one due to steric interactions. Therefore, the epoxidation of both styrenes by the Shi catalyst will be stereospecific and retains all the stereochemical information of the reactant. However, whether or not (R)-styrene oxide or (S)-styrene oxide is form is dependent on the relative energies of the transition state. The more stable the transition state, the more likely the product of the transition state will be formed.
So in order to examine this, an example of transition state of both styrenes with Shi catalyst are obtained and analyzed.
| Free Energy of (R,R) transition state | Free Energy of (S,S) transition state | Difference in free energies (Hartrees) | K | Enantiomeric Excess (%) |
| -1303.736813 | -1303.727673 | -0.00914 | 6.28E-05 | 99.99 |
From the above, we can see the predicted high enantiopurity of the reaction favoring the (R) product because of the lower energy of the transition states. To understand the reason, we have to turn to non-covalent interaction (NCI) analysis.
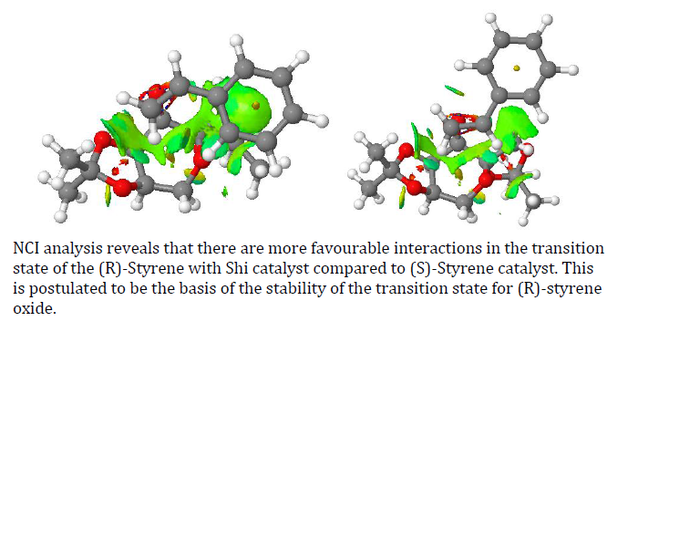
To complement the NCI analysis, a QTAIM (Quantum Theory of Atoms in Molecules) analysis is conducted as well.
| (R)-styrene transition state | (S)-styrene transition state |
 |
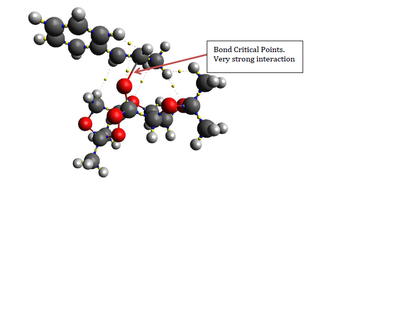 |
| Comments | |
| Unsurprisingly, QTAIM analysis has also shown that (R)-styrene/Shi catalyst has more interactions with one another. While (R)-styrene has 2 strong interactions, (S)-styrene has only one with the catalyst. Besides, the phenyl ring of (R)-styrene also confers additional positive interaction with the catalyst. QTAIM analysis has also show several interactions between the C=C geminal hydrogen of (R)-styrene intreacting with the oxygen atoms on the dioxacyclopentane ring | |
(R,R) and (S,S)-Stilbene transition state
| (R,R)-Stilbene oxide | (S,S)-Stilbene oxide | |||||||
| Structure of Transition State |
|
| ||||||
| Approach of ligand | 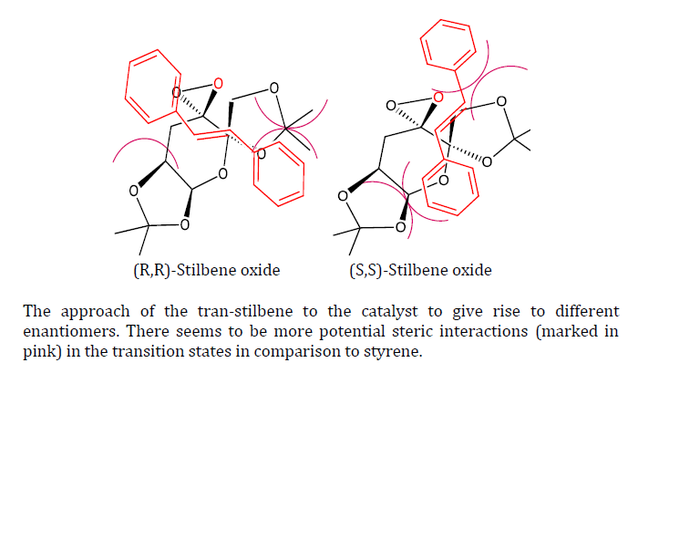 | |||||||
However, unlike styrene, trans-stilbene seems to approach perpendicularly to the dioxirane. This is proposed to be due to the steric interactions (marked in purple). Based on first inspection, it seems that (S,S)-Stilbene oxide has more steric interactions than (R,R)-Stilbene oxide. Although unsubstantial, it seems like my guess was correct. The (R,R)-stilbene/Shi catalyst transition state is lower in energy.
| Free Energy of (R,R) transition state | Free Energy of (S,S) transition state | Difference in free energies (Hartrees) | K | Enantiomeric Excess (%) |
| -1534.687808 | -1534.683440 | 0.004368 | 9.08E-3 | 98.20 |
NCI Analysis is also done for trans-stilbene
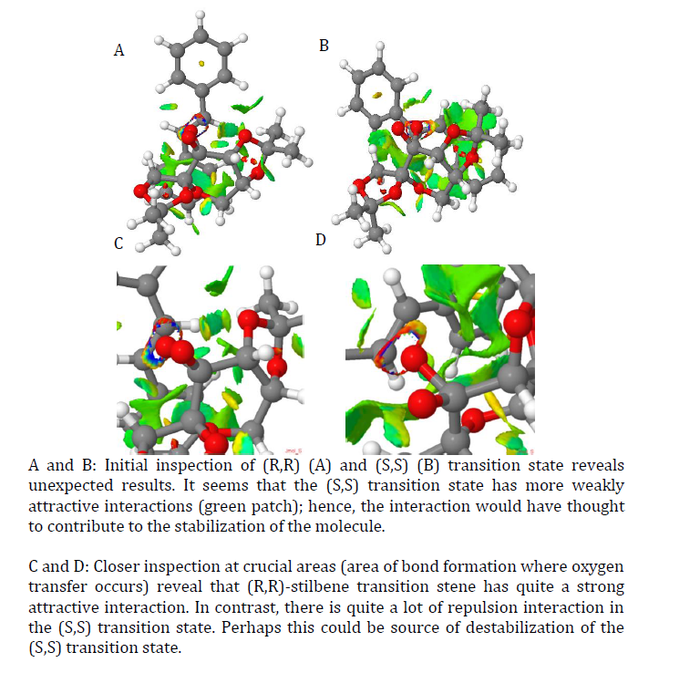
Jacobsen Catalyst
For this section, cis-beta methyl styrene transition state will be analyzed.
| Free Energy of (R,R) transition state | Free Energy of (S,S) transition state | Difference in free energies (Hartrees) | K | Enantiomeric Excess (%) |
| -3383.259559 | -3383.251060 | -0.008499 | 1.24E-04 | 99.98 |
| -3383.253442 | -3383.250270 | -0.003172 | 3.48E-02 | 93.27 |
As is obvious from here, the (S,R) transition state is more stable than the (R,S) transition state. Both transition states are exo with respect to the catalyst.
It is not immediately apparent just by looking at the molecule to determine which spatial groups have interactions with one another. Therefore, an NCI analysis is conducted again.

Bibliography
1. David C. Forbes, Sampada V. Bettigeri,Samit A. Patrawala, Susanna C. Pischek, and Michael C. Standen, Tetrahedron, 2009, 65(1), 70-76
2. Hui Lina, Jing Qiaoa, Yan Liua, Zhong-Liu Wu, Journal of Molecular Catalysis B: Enzymatic, 2010, 67, 236-241
3. Niwa,Takashi, Nakada,Masahisa, Journal of the American Chemistry , 2012, 134, 13538-13541
4. Arlette Solladié-Cavallo, Anh Diep-Vohuule, Tetrahedron: Asymmetry, 1996, 7(6), 1783-1788
5. Vito Capriati, Saverio Florio, Renzo Luisi,Filippo Maria Perna, Antonio Salomone, Francesco Gasparrini, Org. Lett., 2005, 7 (22), 4895-4898
6. Wong O.Andrea, Wang Bin, Zhao Mei-Xin, Shi Yian, Journal of Organic Chemistry, 2009, 74, 6335-6338
7. Alan B. McEwen, Paul v. R. Schleyer, Journal of The American Chemical Society, 2002, 108(4), 250-253
8. Halgren T. A. J. Comput. Chem., 1999, 20, 720-729.
9. J. Hanson,J. Chem. Educ., 2001, 78, 1266