Rep:Mod:Computational Y2 amm416
EX3
BH3
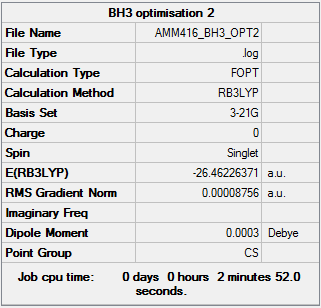
3-21G Pre-optimisation Calculation
Item Value Threshold Converged? Maximum Force 0.000217 0.000450 YES RMS Force 0.000105 0.000300 YES Maximum Displacement 0.000919 0.001800 YES RMS Displacement 0.000441 0.001200 YES Predicted change in Energy=-1.635268D-07 Optimization completed. -- Stationary point found.
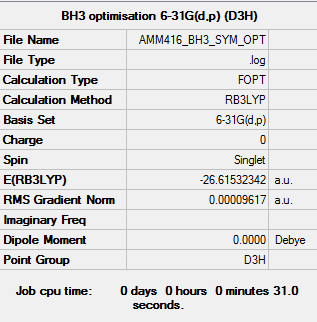
6-31G(d,p) (D3h symmetry constraint applied) Optimisation Calculation
Item Value Threshold Converged? Maximum Force 0.000192 0.000450 YES RMS Force 0.000126 0.000300 YES Maximum Displacement 0.000763 0.001800 YES RMS Displacement 0.000500 0.001200 YES Predicted change in Energy=-2.201780D-07 Optimization completed. -- Stationary point found.
Frequency Calculation
BH3 Frequency Calculation .log File
Low frequencies --- -0.2263 -0.1037 -0.0054 47.9770 49.0378 49.0383 Low frequencies --- 1163.7209 1213.6704 1213.6731
BH3 |
IR of BH3


In the IR of BH3, only 3 peaks are present even though there are 6 vibrations. As seen in the Table of IR frequencies, there are 2 sets of 2 generate vibrations: odes 2 and 3 are degenerate (scissoring and rocking) as well as modes 5 and 6 (both asymmetric stretches). Therefore, the degenerate signals will overlap forming a single peak, generating two peaks in the spectrum. The third peak is due to the wagging motion (mode 1). The remaining peak would be the one due to the symmetric stretching, but in this vibrational mode there is no change in net dipole moment; therefore, it is IR inactive.
MO Diagram

The computed MOs are very similar to the qualitative LCAO analysis. The only MO that is not immediately recongnisable is one of the 2e' antibonding orbitals; the computed MO on the left shows more lobe repulsions than predicted in the qualitative LCAO. Nevertheless, predicting MO shapes and relative sizes qualitatively by linearly combining the AOs gives a very good approximation of the real MOs.
Smf115 (talk) 23:29, 16 May 2018 (BST)Great comment, picking up on the subtle differences and on the good approximation of qualitative MO theory.
Association Energies: Ammonia-Borane
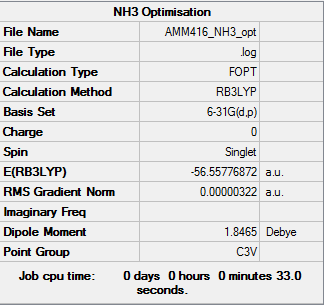
NH3 Optimisation and Frequency Calculation
Optimisation
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000016 0.001800 YES RMS Displacement 0.000011 0.001200 YES Predicted change in Energy=-1.228228D-10 Optimization completed. -- Stationary point found.
Frequency
NH3 Frequency Calculation .log File
Low frequencies --- -0.0138 -0.0032 -0.0015 7.0783 8.0932 8.0937 Low frequencies --- 1089.3840 1693.9368 1693.9368
NH3 |
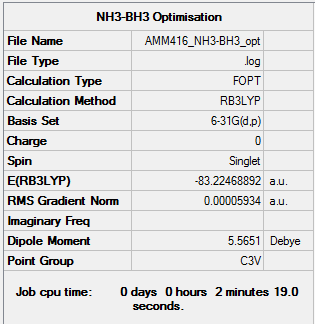
NH3-BH3 Optimisation and Frequency Calculation
Optimisation
Item Value Threshold Converged? Maximum Force 0.000121 0.000450 YES RMS Force 0.000057 0.000300 YES Maximum Displacement 0.000505 0.001800 YES RMS Displacement 0.000294 0.001200 YES Predicted change in Energy=-1.610954D-07 Optimization completed. -- Stationary point found.
Frequency
NH3BH3 Frequency Calculation .log File
Low frequencies --- -0.0252 -0.0033 -0.0012 17.0405 17.0427 36.9265 Low frequencies --- 265.7534 632.2124 639.3376
NH3-BH3 |
Association Energy Calculations
E(NH3)= -26.61532 a.u.
E(BH3)= -56.55777 a.u.
E(NH3BH3)= -83.22469 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)], where ΔE is the dissociation energy of NH3BH3
∴ ΔE = -0.05160 a.u. = - 135 kJmol-1
Hence, the N-B bond energy is 135 kJmol-1. This value is 3 times smaller than the energy of the C-C bond in the corresponding molecule of ethane, 402 kJmol-1.
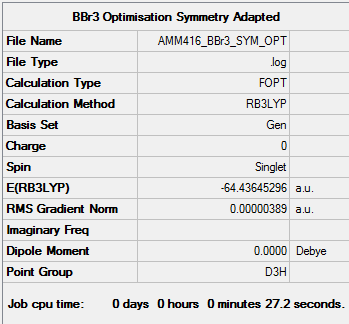
BBr3 Optimisation and Frequency Calculation using SCAN Server
Optimisation
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000024 0.001200 YES Predicted change in Energy=-4.190601D-10 Optimization completed. -- Stationary point found.
Frequency
BBr3 Frequency Calculation .log File
Low frequencies --- -0.0136 -0.0064 -0.0046 2.4367 2.4367 4.8447 Low frequencies --- 155.9631 155.9651 267.7048
BBr3 |
D-Space Link: DOI:10042/202296
Ionic Liquids: Designer Solvents
Optimisation and Frequency Calculations for [N(CH3)4]+ and [P(CH3)4]+
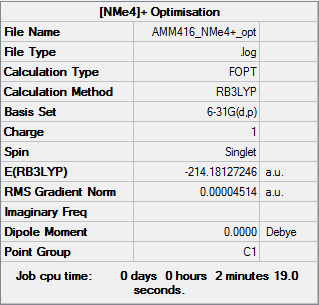
[N(CH3)4]+
Optimisation
Item Value Threshold Converged? Maximum Force 0.000074 0.000450 YES RMS Force 0.000027 0.000300 YES Maximum Displacement 0.000362 0.001800 YES RMS Displacement 0.000111 0.001200 YES Predicted change in Energy=-9.316300D-08 Optimization completed. -- Stationary point found.
Frequency
[N(CH3)4]+ Frequency Calculation .log File
Low frequencies --- -7.5520 -0.0011 -0.0009 0.0003 6.8978 7.9666 Low frequencies --- 184.2924 289.3429 289.8709
[N(CH3)4]+ |
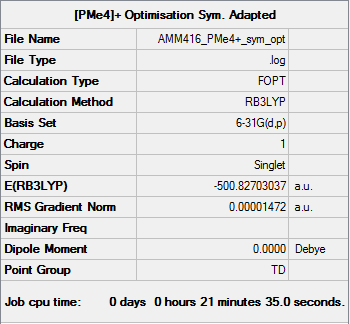
[P(CH3)4]+
Optimisation
Item Value Threshold Converged? Maximum Force 0.000030 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.000107 0.001800 YES RMS Displacement 0.000044 0.001200 YES Predicted change in Energy=-1.742375D-08 Optimization completed. -- Stationary point found.
Frequency
[P(CH3)4]+ Frequency Calculation .log File
Low frequencies --- -0.0032 0.0017 0.0018 25.3058 25.3058 25.3058 Low frequencies --- 161.2512 195.7467 195.7467
[P(CH3)4]+ |
Smf115 (talk) 23:26, 16 May 2018 (BST)Great structure information and inclusion of the charges on the complexes
Charge Distribution Analysis




From Table 2, it can be seen that nitrogen is partially negatively charged as well as the carbon atoms. Therefore, the classical model in which nitrogen in a tetrahedral arrangement has a positive charge is actually wrong. The assumed positive charge arises from the fact that nitrogen can form a dative covalent bond and hence become more electrophilic. However, from the charge distribution analysis, the positive charge is entirely located on the hydrogen atoms.
In [P(CH3)4]+, all the H atoms and the central P atom are partially positive, with the C atoms bearing all the negative charge, as seen from Table 3. Compared to N in [N(CH3)4]+, P is positively charged because its electronegativity is lower than N's, hence attracts less electron density.
Smf115 (talk) 23:25, 16 May 2018 (BST)Good discussion which could have been improved by considering the C and H charges, particularly the similarity of the charges on H across the molecule.
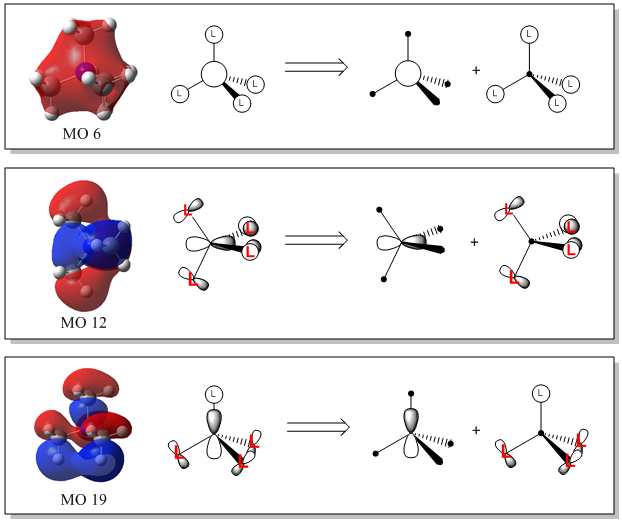
MO Analysis of [N(CH3)4]+
Smf115 (talk) 22:54, 16 May 2018 (BST)Good attempt at the LCAOs and ligand FOs however, consideration of the BH3 orbitals seen in your lectures and how these relate to the CH3 FO's for the ligands was needed.
Smf115 (talk) 23:34, 16 May 2018 (BST)Overall a good report and a well presented project section with some further thought on the FOs and LCAOs required.