Rep:Mod:Clarachong
NH3 molecule
General Information
| NH3 Ammonia | ||||
|---|---|---|---|---|
| Calculation Type | OPT, FREQ | |||
| Calculation Method | RB3LYP | |||
| Basis Type | 6-31G(D,P) | |||
| RMS gradient (a.u.) | 0.00000485 | |||
| Final energy E (a.u.) | -56.5577687 | |||
| Point group | C3v | |||
| N-H bond length | 1.018 | |||
| N-H bond angle | 105.74 | |||
Obtain the optimisation file here
Optimising the molecule: Item table
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy=-5.986274D-10
Vibrational modes of NH3
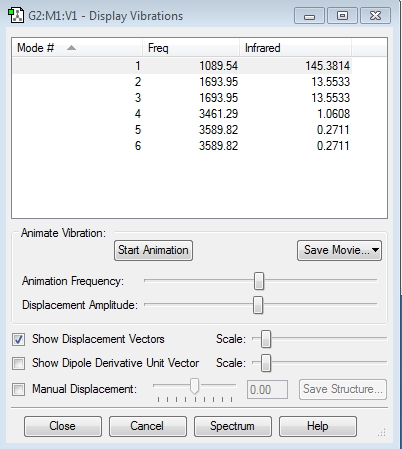
| Expected number of modes | 3N-6 = 3(4)-6 =6 |
| Number of degenerate modes | Modes 2 and 3 Modes 5 and 6 |
| "Bending" vibrations | Modes 1,2,3 |
| "Bond stretch" vibrations | Modes 4,5,6 |
| Umbrella mode | Mode 1 |
| Highly symmetric mode | Mode 4 |
| Number of bands expected to be observed | Even though there are 4 distinct modes, only 2 of them (Mode 1 and degenerate modes 2 and 3) will be observed. This is because the IR intensity of modes 4,5 and 6 (reflected on the right most row) are too low to be seen and will disappear on the IR spectrum. |
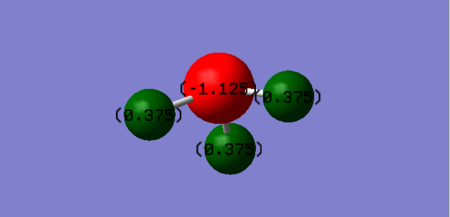
Atomic charges of NH3
| Atom | Charge |
|---|---|
| Hydrogen | +0.375 |
| Nitrogen | -1.125 |
Nitrogen is expected to carry a negative charge as it is more electronegative compared to the neighbouring Hydrogen atoms. Hence, due to Nitrogen's larger electronegativity value, the electrons will be situated nearer the Nitrogen atom, causing it to take on a negative charge.
N2 molecule
General Information
| N2 molecule | ||||
|---|---|---|---|---|
| Calculation Type | OPT, FREQ | |||
| Calculation Method | RB3LYP | |||
| Basis Type | 6-31G(D,P) | |||
| RMS gradient (a.u.) | 0.00000060 | |||
| Final energy E (a.u.) | -109.52412868 | |||
| Point group | D∞h | |||
| N-H bond length | 1.10550 | |||
Obtain the optimisation file link here
Optimising the molecule: Item table
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.401076D-13
Vibrational modes of N2
| Frequency | Infrared |
|---|---|
| 2457.33 | 0.0000 |
Since both nitrogen atoms are identical, the molecule does not have a dipole moment and hence will not be detected by IR spectroscopy, giving a infrared reading of zero.
Atomic charges of N2
| Atom | Charge |
|---|---|
| Nitrogen | 0.000 |
Since both Nitrogen atoms are identical and indistinguishable, both atoms do not carry any charge.
Molecular orbitals of N2
When 2 atoms react to form a molecule, the number of molecular orbitals (MO) in the molecule will be a linear combination of the number of atomic orbitals (AO) of each atom. The total number of electrons in the MO diagram will be the total number of electrons of each atom. Since Nitrogen has a 7 electrons with an electronic configuration of 1s22s22p3, the N2 molecule has a total of 14 electrons, with each p electron involved in 1 sigma and 2 pi bonds.
Reactions often involve the highest energy electrons, located in the highest energy molecular orbital (HOMO). Electrons that are donated to the molecule will be located at the lowest possible energy level, known as the lowest energy unoccupied molecular orbital (LUMO). Shown in the table below are diagrams of the HOMO and LUMO of N2.
| HOMO | LUMO |
|---|---|
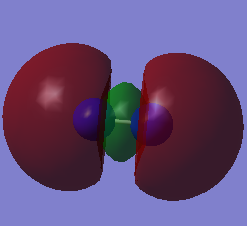 |
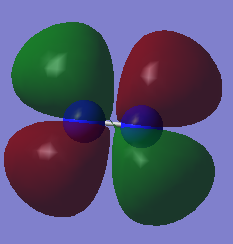
|
H2 molecule
General Information
| H2 molecule | ||||
|---|---|---|---|---|
| Calculation Type | OPT, FREQ | |||
| Calculation Method | RB3LYP | |||
| Basis Type | 6-31G(D,P) | |||
| RMS gradient (a.u.) | 0.00000017 | |||
| Final energy E (a.u.) | -1.17853936 | |||
| Point group | D∞h | |||
| N-H bond length | 1.10550 | |||
Obtain the optimisation file link here
Optimising the molecule: Item table
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-1.164080D-13
Vibrational modes of H2
| Frequency | Infrared |
|---|---|
| 4465.68 | 0.0000 |
Similar to N2, H2 is not IR active as it does not have a dipole moment and no change in dipole is observed in the symmetric stretch.
Comparing both H2 and N2 molecules, it can be observed that the frequency of the H2 symmetric stretch is higher. Frequency of vibration is inversely proportional to the reduced mass of the molecule and proportional to bond strength.
| Molecule | Bond strength/KJmol-1 [1] | Reduced mass/a.u. |
|---|---|---|
| H2 | 436 | 0.5 |
| N2 | 945 | 7 |
It can be observed from the table above that the bond strength of N2 is around twice of H2 as N2 has a bond order of 3 (triple bond) compared to H2 which only has a bond order of 1 (single bond). However, N2 has a reduced mass 14 times that of H2. The effect due to the difference in reduced mass outweighs that of bond strength. Since reduced mass and frequency of vibration are inversely proportional, the stretching frequency of N2 is much lower.
Atomic charges of H2
| Atom | Charge |
|---|---|
| Hydrogen | 0.000 |
Since both Hydrogen atoms are identical and indistinguishable, both atoms do not carry any charge.
Molecular orbitals of H2
Similar to the MO diagram shown for N2, the table below shows the HOMO and LUMO for H2. Since Hydrogen has an electronic configuration of 1s1, there will only be 2 electrons in the MO diagram, where the sigma bond is formed.
| HOMO | LUMO |
|---|---|
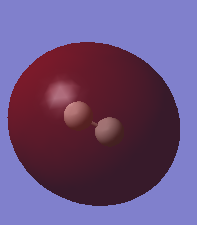 |
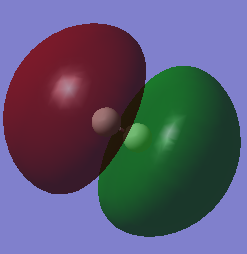
|
The Haber-Bosch process
N2 + 3H2 2NH3
All data recorded in the table below is in a.u.
| E(NH3) | -56.55776873 |
| 2*E(NH3) | -113.11553746 |
| E(N2) | -109.52412868 |
| E(H2) | -1.17853936 |
| 3*E(H2) | -3.53561808 |
| ΔE=2*E(NH3)-[E(N2)+3*E(H2)] | -0.0557907 |
Hence, ΔE (KJmol-1) = -0.0557907 x 2625.5 = -146.4784829 KJmol-1
The negative sign on ΔE indicates that energy is being released into the surroundings during the formation of ammonia from nitrogen and hydrogen. This suggests that the products (ammonia) are lower in energy than the reactants, and hence indicating that they are more thermodynamically stable. The forward reaction which produces ammonia would be favoured.
CH4 molecule
General Information
Obtain the optimisation file here
Optimising the molecule: Item table
Item Value Threshold Converged? Maximum Force 0.000063 0.000450 YES RMS Force 0.000034 0.000300 YES Maximum Displacement 0.000179 0.001800 YES RMS Displacement 0.000095 0.001200 YES Predicted change in Energy=-2.256037D-08
Vibrational modes of CH4
| CH4 Methane | ||
|---|---|---|
| Mode 1 | Mode 2 | Mode 3 |

|
|
|
| Frequency: 1356.20Hz, Infrared: 14.1008 | Frequency: 1356.20Hz, Infrared: 14.1008 | Frequency: 1356.20Hz, Infrared: 14.1008 |
| Modes 1, 2 and 3 are degenerate bending modes in 3 different orientations (x, y and z axis) | ||
| Mode 4 | Mode 5 | Mode 6 |
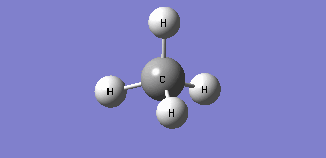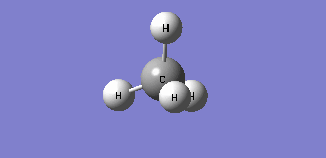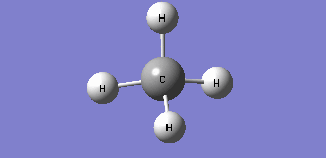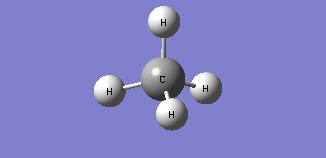
|
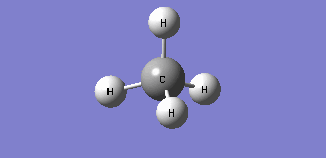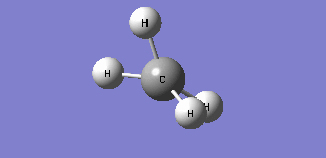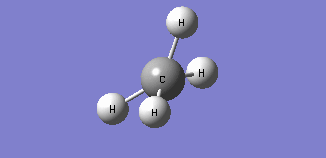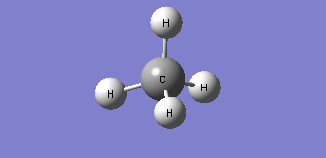
|
|
| Frequency: 1578.58Hz, Infrared: 0 | Frequency: 1578.58Hz, Infrared: 0 | Frequency: 3046.46Hz, Infrared: 0 |
| Modes 4 and 5 are degenerate bending modes while mode 6 is a symmetric stretching. Since all 3 modes do not result in a change in dipole moment, they are not IR active. | ||
| Mode 7 | Mode 8 | Mode 9 |
|
|
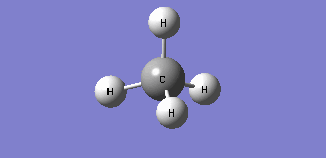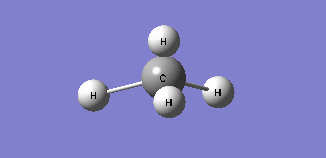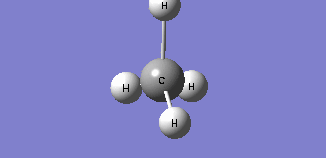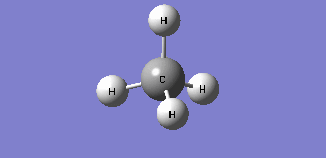
|
| Frequency: 3162.33Hz, Infrared: 25.3343 | Frequency: 3162.33Hz, Infrared: 25.3343 | Frequency: 3162.33Hz, Infrared: 25.3343 |
| Modes 7, 8 and 9 are asymmetric stretching modes which are degenerate. | ||
| It can be observed that stretching vibrations usually occur at higher infrared intensity levels around 25 compared to bending vibrations at around 14. Since it is easier to bend a bond compared to compressing or stretching it, stretching vibrations occur at higher energy levels and hence result in higher intensity IR readings. | ||
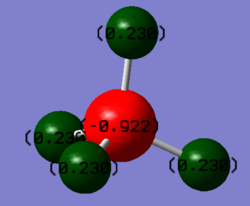
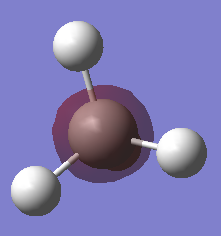
Atomic charges of CH4
| Atom | Charge |
|---|---|
| Carbon | -0.922 |
| Hydrogen | 0.230 |
As seen from the screenshot above, more electron density can be found on the carbon instead of the hydrogens, causing carbon to take on a partial negative charge. This is because carbon is more the electronegative element. Since methane is a neutral molecule, it can be observed that the sum of the partial positive charges on all the hydrogens are similar to the partial negative charge on carbon.
Molecular orbitals of CH4
| Molecular Orbital | Energy | Comments | MO diagram [2] |
|---|---|---|---|
 |
-10.16707 | MO1, the lowest energy 1s bonding orbital surrouding only the central carbon atom. It is not drawn in the MO diagram as it is too low in energy. They contain the two 1s electrons from the carbon atom. | 
|
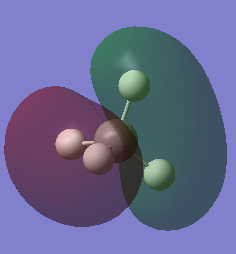 |
-0.38831 | MO3, MO4 and MO5 represent 3 degenerate bonding HOMOs. They represent the bonding 2s orbitals in 3 different orientations (x, y and z axis). They are contributed by the 1s orbitals of the four hydrogen atoms and 2s orbital of the carbon atom. | |
 |
0.11842 | MO6 represents the LUMO. MO6 is the empty antibonding orbital of MO2. Both MO2 and MO6 are spherical in shape, with MO6 comparatively larger, hence higher in energy. | |
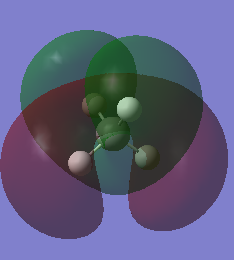 |
0.17677 | MO7, MO8, MO9 represent three degenerate antibonding molecular orbitals in three different orientations. They are antibonding orbitals for bonding orbitals MO3, MO4 and MO5. Compared to MO10, it has fewer nodal regions, hence lower in energy. | |
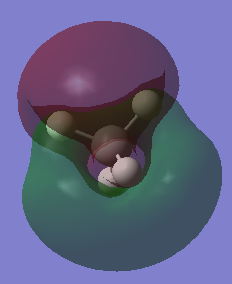 |
0.52915 | MO10 is a high energy antibonding orbital, which is degenerate with MO11 and MO12. (not shown in MO diagram) |
CH3̹+ and CH3̹- ions
General Information
| CH3̹+ carbocation | CH3̹- carboanion | ||||||
|---|---|---|---|---|---|---|---|
|
| ||||||
| Calculation Type | OPT, FREQ | Calculation Type | OPT, FREQ | ||||
| Calculation Method | RB3LYP | Calculation Method | RB3LYP | ||||
| Basis Type | 6-31G(D,P) | Basis Type | 6-31G(D,P) | ||||
| RMS gradient (a.u.) | 0.00010036 | RMS gradient (a.u.) | 0.00010504 | ||||
| Final energy E (a.u.) | -39.48468012 | Final energy E (a.u.) | -39.79602794 | ||||
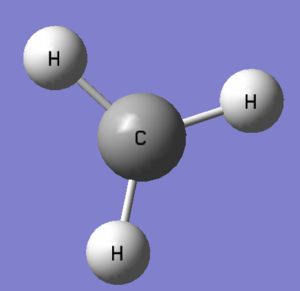
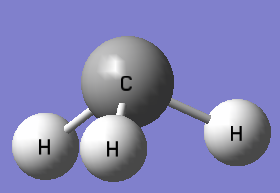
| Point group | D3H | Point group | C3V | ||||
| Dipole Moment | 0 | Dipole Moment | 1.7169 | ||||
| Obtain the optimisation file link here | Obtain the optimisation file link here | ||||||
| According to the VSEPR theory, the electron deficient postively charged carbocation (3 bond pairs and no lone pairs) will adopt a trigonal planar symmetry shown above. | Unlike the carbocation, the presence of an additional lone pair of electrons on CH3̹- will result in a trigonal pyramidal symmetry (similar to ammonia) | ||||||
Optimising the molecule: Item table
CH3̹+
Item Value Threshold Converged? Maximum Force 0.000201 0.000450 YES RMS Force 0.000131 0.000300 YES Maximum Displacement 0.000569 0.001800 YES RMS Displacement 0.000373 0.001200 YES Predicted change in Energy=-1.714129D-07
CH3̹-
Item Value Threshold Converged? Maximum Force 0.000173 0.000450 YES RMS Force 0.000114 0.000300 YES Maximum Displacement 0.000523 0.001800 YES RMS Displacement 0.000372 0.001200 YES Predicted change in Energy=-1.736478D-07