Rep:Mod:CTL0218
NH3
Key Information
| Molecule name | Ammonia |
| Molecular Formula | NH3 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -56.55640542 |
| RMS gradient | 0.00980112 |
| Point Group | C3V |
| Bond Length | 1.04 Å |
| Bond Angle | 102° |
| Item | Value | Threshold | Converged? | |
|---|---|---|---|---|
| Maximum | Force | 0.000004 | 0.000450 | YES |
| RMS | Force | 0.000004 | 0.000300 | YES |
| Maximum | Displacement | 0.000072 | 0.001800 | YES |
| RMS | Displacement | 0.000035 | 0.001200 | YES |
test molecule |
Vibrations
| Wavenumber (cm-1) | 1090 | 1694 |
| Symmetry | A1 | E |
| Intensity (arbitrary units) | 145 | 14 |
| Image | 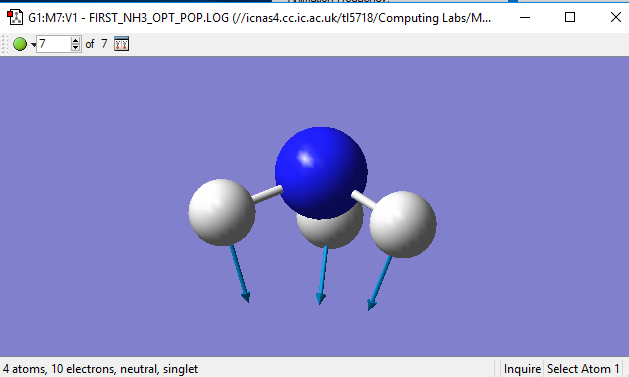 |

|
Using the 3N-6 rule, 6 vibrational modes are expected as the molecule is non-linear. Modes 2 and 3, and 5 and 6 are degenerate as they have the same energy. Modes 2,3 and 5 are "bending" vibrations whereas modes 1,4 and 6 are " bond stretch" vibrations where mode 1 is known as the "umbrella" mode. Consequently, it would be expected to see 4 bands in an experimental spectrum of gaseous ammonia.
Charges
| Atom | Charge |
|---|---|
| N | -1.125 |
| H | 0.375 |
A negative charge is expected for the N atom while a postive value is expected for the H atom; this is because N is more electronegative than H therefore is able to attract more electrons so has a larger electron density.
Nitrogen
Key Information
| Molecule name | Nitrogen |
| Molecular Formula | N2 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -109.52359111 |
| RMS gradient | 0.02473091 |
| Point Group | D*H |
| Bond Length | 1.09 Å |
| Bond Angle | 180° |
| Item | Value | Threshold | Converged? | |
|---|---|---|---|---|
| Maximum | Force | 0.000001 | 0.000450 | YES |
| RMS | Force | 0.000001 | 0.000300 | YES |
| Maximum | Displacement | 0.000000 | 0.001800 | YES |
| RMS | Displacement | 0.000000 | 0.001200 | YES |
test molecule |
Vibrations
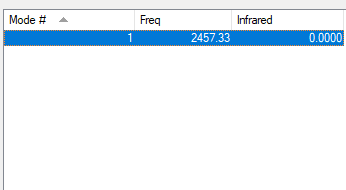
| Wavenumber (cm-1) | 2457 |
| Symmetry | SGG |
| Intensity (arbitrary units) | 0.00 |
Charges
| Atom | Charge |
|---|---|
| N | 0.000 |
Hydrogen
Key Information
| Molecule name | Hydrogen |
| Molecular Formula | H2 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -1.15928020 |
| RMS gradient | 0.09719500 |
| Point Group | D*H |
| Bond Length | 0.60 Å |
| Bond Angle | 180° |
| Item | Value | Threshold | Converged? | |
|---|---|---|---|---|
| Maximum | Force | 0.000000 | 0.000450 | YES |
| RMS | Force | 0.000000 | 0.000300 | YES |
| Maximum | Displacement | 0.000000 | 0.001800 | YES |
| RMS | Displacement | 0.000001 | 0.001200 | YES |
test molecule |
Vibrations
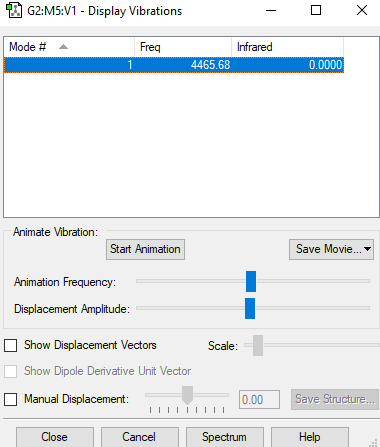
| Wavenumber (cm-1) | 4466 |
| Symmetry | SGG |
| Intensity (arbitrary units) | 0.00 |
Charges
| Atom | Charge |
|---|---|
| H | 0.000 |
Transition metal complex containing N2
Unique Identifier: DAYSUR n2_metalcomplex.cqs N2 bond length in the structure: 1.04Å The crystal structure distance is less than that of the computational distance of 1.09Å, this is due to the effects of the other ligands around the central metal atom that causes partially ionic character.
Energies
| E(NH3) | -56.6 a.m.u |
| 2*E(NH3) | -113 a.m.u |
| E(N2) | -110 a.m.u |
| E(H2) | -1.16 a.m.u |
| 3*E(H2) | -3.48 a.m.u |
| ΔE=2*E(NH3)-[E(N2)+3*E(H2)] | 0.48 a.m.u |
Energy to convert H2 and N2 into NH3 gas( N2 + 3H2 -> 2NH3)= 1260 kJ/mol where the product is more stable.
Carbon Dioxide
Key Information
| Molecule name | Carbon Dioxide |
| Molecular Formula | CO2 |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d.p) |
| Final Energy | -188.55450992 |
| RMS gradient | 0.0665368 |
| Point Group | D*H |
| Bond Length | 1.26 Å |
| Bond Angle | 180° |
| Item | Value | Threshold | Converged? | |
|---|---|---|---|---|
| Maximum | Force | 0.000024 | 0.000450 | YES |
| RMS | Force | 0.000017 | 0.000300 | YES |
| Maximum | Displacement | 0.000021 | 0.001800 | YES |
| RMS | Displacement | 0.000015 | 0.001200 | YES |
test molecule |
Vibrations
| Wavenumber (cm-1) | 2436 |
| Symmetry | SGU |
| Intensity (arbitrary units) | 546 |
Charges
| Atom | Charge |
|---|---|
| C | 1.022 |
| O | -0.511 |
Molecular Orbitals
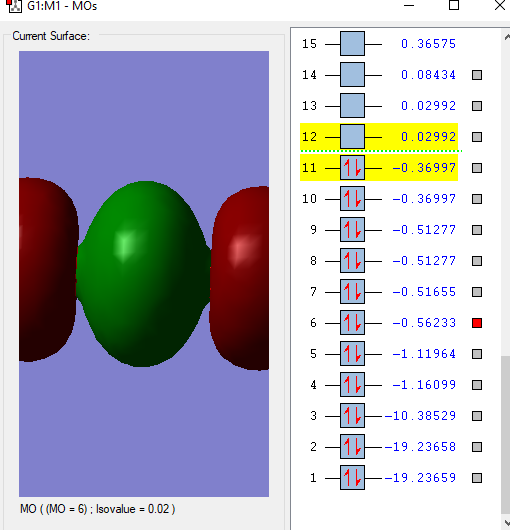
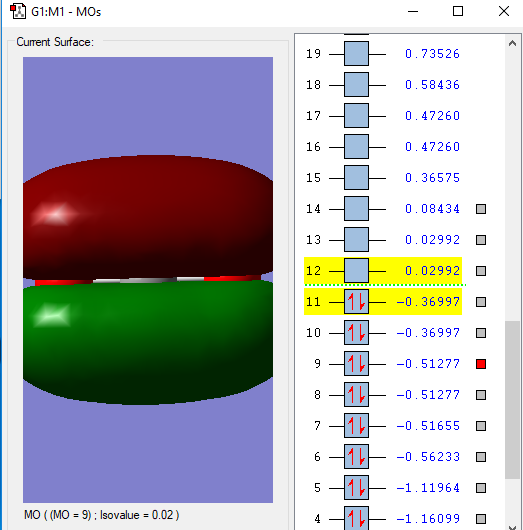
The Carbon and Oxygen atoms each have a 2s orbital and a 2p orbital that is split into px,py and pz. These atomic orbitals undergo linear combination to form molecular orbitals which correspond to certain energies.
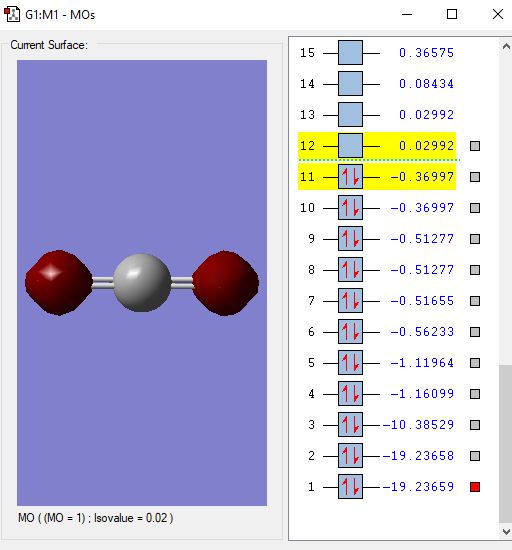
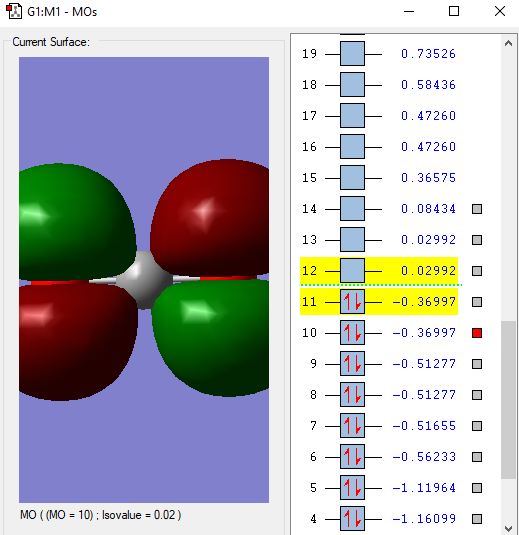
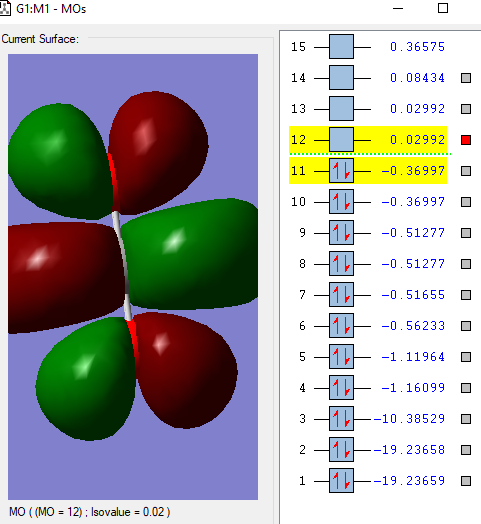
This is the lowest energy molecular orbital that is the 1σg and contains a pair of electrons from an O atom.
This molecular orbital,2σg, is formed from the combination of the 2s σg orbital from the C and one of the 2p orbitals.
This 1πu molecular orbital is formed from the combination of a 2p πu orbital in C and a 2p orbital in O. There are 2 of this orbital that are degenerate as there are 2 p atomic orbitals on each atom that are perpendicular to the bond combining so they are of the same energy.
There are also 2 of this 1πg anti bonding molecular orbital that is formed due to the electrons in p orbital of the O atom. This is the HOMO(Highest Occupied Molecular Orbital).
This is the LUMO(Lowest Unoccupied Molecular Orbital), the 2πu antibonding orbital formed from a 2p πu orbital in C and a 2p orbital in O.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 0.5/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
Overall good presentation however you have used the same default captions for all of your jmols which is not helpful to a readed. Also your MO pictures are cut off by the borders of the window. You should have resized the windows or zoomed out (using mouse wheel).
NH3 0/1
Have you completed the calculation and given a link to the file?
The link to your .log file is broken. Also you should include your username in all uploads to the wiki system as otherwise you risk overwriting other people's files.
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES, although your H-N-H angle is incorrect, should be 106 not 102.
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES, However you missed out one question in the vibrational section. Also there are only 2 visible peaks in the spectra of NH3, due to the low intensity of the other 2 peaks. (See infrared column in vibrations table.)
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES, but you have not included links to your .log files.
Also you have given a bond angle of 180 for N2 and H2, there are no bond angles in diatomic molecules. Bond angles involve exactly 3 atoms.
Crystal structure comparison 0/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
No you did not link to the structure.
Have you compared your optimised bond distance to the crystal structure bond distance?
YES, you made a good effort to compare the bond distances, well done.
Haber-Bosch reaction energy calculation 0/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
No
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
No
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 2/5
Have you completed the calculation and included all relevant information?
YES, as you have not included a link to the .log file the highest mark you can recieve is reduced to 4.
Have you added information about MOs and charges on atoms?
YES you have added some pictures of MOs and the charge values. You could have improved by offereing an explanation for the charge values, and by discussing the MOs in more detail.
Independence 0/1
If you have finished everything else and have spare time in the lab you could:
Check one of your results against the literature, or
Do an extra calculation on another small molecule, or
Do some deeper analysis on your results so far
No independent work was found.