Rep:Mod:CSWmodule1
Molecular Mechanics and Semi-Empirical Molecular Orbital methods for Structural and Spectroscopic Evaluations
Introduction
Modelling using Molecular Mechanics
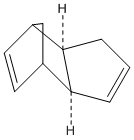
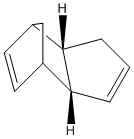
Hydrogenation of Cyclopentadiene Dimers
It is known that Cyclopentadiene dimerises to the endo dimer Dicyclopentadiene at room temperature via the Diels-Alder reaction, see reaction scheme below.
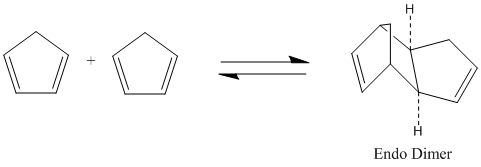
This Pericyclic reaction proceeded via a 4пs + 2пs cycloaddition reaction to form the kinetic product.
The following analysis can be used to explain why the endo cyclopentadiene dimer is more stable than the exo cyclopentadiene dimer. It can also be used to predict whether the observed cyclodimerisation and hydrogenation of cyclopentadiene would be under thermodynamic or kinetic control. Using Molecular Modeling Mechanics the energies and geometries of the dimers will be considered. The molecular mechanics model with take into account any bond strain and/or steric hindrance , but no quantum mechanical (orbital) factors will be taken into account here. The energies will indicate which dimer is more thermodynamically stable and thus give information about the reactivity of the dimer.
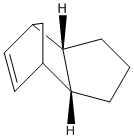
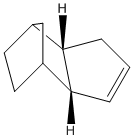
The geometries and energies of the four species, exo dimer 1, endo dimer 2, and dihydro derivatives 3 and 4 (that occur before the dimer is fully hydrogenated to the tetrahydro derivitive, 5) will be calculated.
This will be done using ChemBio3D Ultra where the MM2 energy minimisation calculation will be performed. This model minimally adjusts the geometry of the molecule in order to find the most energetically stable conformer.
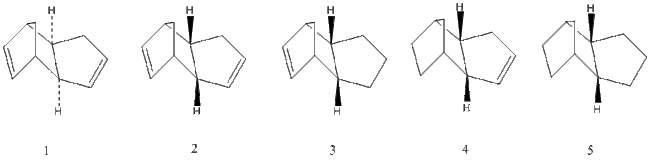
Table of Calculated Energies using MM2
The Dimerisation of Cyclopentadiene Discussion
As seen in the table above, the exo dimer 1, with 31.88 kcal/mol, 1 has a lower energy than the endo dimer, 2 with 33.1 kcal/mol, a difference of 1.22 kcal/mol. This is a significant enough energy difference to lead to dimer 1 being more stable. The results show that this can mainly be attributed to the extra torsion energy in dimer 2 which is 9.51kcal/mol vs 7.65.02kcal/mol in dimer 2.
This molecular modeling process analyses the molecules in terms of thermodynamic stability. Therefore, given that theory and experiment dictate that the endo dimer is exclusively formed we can conclude that this reaction leads to the kinetic product, dimer 2, not the thermodynamic product, dimer 1.
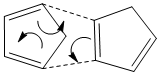
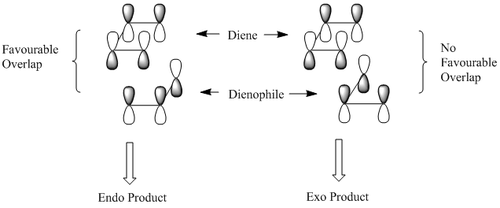
Kinetic products are formed when there is overlap of orbitals in the transition state. Since the MM2 energy minimisation model only takes into account mechanical properties of the molecule, orbital overlap has not been considered here. However, a simple theoretical diagram can be used to exemplify why the kinetic (endo) product is favoured in this dimerisation reaction. [1] . [2]
Hydrogenation of Cyclopentadiene Dimer Discussion
The hydrogenated dimers 3 and 4 are formed form the endo dimer 1. The energy of dimer 3 is 35.68kcal/mol and the energy of dimer is 31.15kcal/mol, which means dimer 4 is the most energetically stable. By looking at the breakdown of energy contributors it can be seen that the bending energy of the hydrogenated dimer 3, 19.86kcal/mol, is significantly greater than that of dimer 4, 14.53kcal/mol, and strongly influences why dimer 4 is the more stable hydrogenated product. This indicates that the bond angles are more favorable in dimer 4, and closer to the desired 120° sp2 geometry of the C-C-C double bond. Dimer 4 actually has more torsional strain energy 12.8 kcal/mol (vs. 10.8 kcal/mol for dimer 3), but this is less significant than the difference in bending energy.
Although the MM2 modeling technique indicates dimer 4 is the most energetically (or thermodynamically) stable product it does not indicate which dimer would actually be formed, since, as above, the kinetic dimer may be formed if the reaction proceeds via a stabilised transition state.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
The two isomers 9 and 10 shown below are key intermediates in the synthesis of Taxol. The sterochemistry of the carbonyl group determines which isomer is most stable. Using ChemBio3D Ultra molecular mechanics MM2 force field the most stable isomer can be determined.
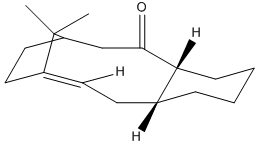
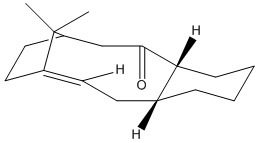
Since the MM2 force field energy minimisation technique only minimally adjusts the conformation of molecules in order to minimise the energy its necessary consider what possible conformations are available, and to then manually move the atoms onto the desired conformation.
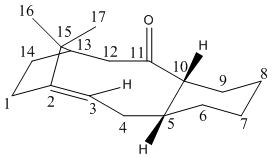
The conformations of the molecule can be analysed by considering the individual conformation of the 3 rings present.
The 5 membered ring, 1, 2, 15, 13, 14, is denoted ring A.
The 9 membered ring, 2, 3, 4, 5, 10, 11, 12, 13, 15 is denoted ring B.
The 6 membered ring, 5, 6 ,7, 8, 9, 10 is denoted ring C.
Ring A will only exist in 1 stable conformation, since the only stable conformation for 5 memebered rings is ‘puckered’. Therefore it is not possible to change the conformation of this ring in order to minimise the energy of the molecule.
The different conformations of the 9-membered ring will affect the energy of the intermediate. In this molecule, the ring is fairly ridged since carbon atoms 2 and 13 are held in place by the carbon 2, 15, 13 bridge. The double bond also extends this rigidity to carbon atom 3. The large carbonyl group and 6 membered ring on carbons 5, 10 and 11 also restrict the available conformations of this ring. After analysis using the MM2 force field energy minimisation, it was deduced that the conformation of ring B is almost entirely influenced by the conformation of ring C. The position of carbons 5 and 10 will have a dramatic influence on the position of the other 7 carbon atoms in ring B. It was therefore discovered that the best way to minimise the energy of the molecule was via positioning ring C in its different conformations. These are chair, half chair, twist boat and boat. The following molecular energies were recorded.
Table of Calculated Energies using MM2
| Conformation | Isomer 9 Energy kcal/mol | Isomer 9 Image | Isomer 10 Energy kcal/mol | Isomer 10 Image |
| Twist Boat | 53.79 |  | 53.63 |  |
| Chair | 47.84 |  | 43.31 |  |
After extensive manipulation the only stable isomers for both and were found with ring C in either twist boat or chair conformation. For both isomers 9 and 10 the most stable conformers were found to contain ring C in chair conformation.
Of the two isomers the most stable isomer was found to be isomer 10, with a minimum energy calculated to be 43.3104kcal/mol vs. 47.84kcal/mol for isomer 9 (A difference of 4.53 kcal/mol. As seen in the figures above, this is the isomer with the carbonyl group pointing down.
Both of these isomers have unusual stability due to alkene hyper conjugation. It is generally considered that alkenes have higher strain energy than alkanes. However, in a number of alkenes the strain energy is actually less than the corresponding alkane to do an increase in transannular interactions between hydrogens. This results in the alkenes being unusually stable and reluctant to undergo hydrogenation reactions. [3]
This behavior is often found in bridgehead alkenes where the geometry of the parent hydrocarbon structure is influenced by substituents. [4] The “olefin strain” energy as it is termed in this paper is defined here as 'the difference between the strain energy of an olefin and that of its parent hydrocarbon'. The hyperstability arises when the olefins are less strained than the parent hydrocarbon and thus less reactive due to the bridgehead location of the double bonds.
Modelling using Semi-empirical Molecular Orbital Theory
Regioselevtive Addition to Dichlorocarbene
Dichlorocarbene is a valuble momomer that can be polymerised to synthetic rubber materials.
Using semi-empirical molecular orbital theory, orbital effects can be considered. This method considers more realistically how the electronic interactions in molecules determine its behavior and characteristics.
This is done in ChemBio3D Ultra by first running the MM2 energy minimisation calculation to tidy up the geometry of the molecule, and then running the MOPAC/PM6 calculation to determine an approximation for the valence-electron molecular wavefunction. Performing this calculation enables a molecular orbital representation to be drawn on the molecule. The HOMO -1, HOMO, LUMO, LUMO +1 and LUMO +2 orbitals were drawn, the results can be seen below:
| HOMO -1 | HOMO | LUMO | LUMO +l | LUMO +2 |
 |  |  | 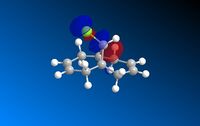 |  |
The observed HOMO and LUMO orbitals over the double bonds indicate which bond is more electrophilic.
The HOMO contains the highest energy electrons that would theoretically enable the molecule to act as a nucleophile. This can be determined by using the molecular orbitals to see which double bond the HOMO lies over. It can be seen from the HOMO orbital diagram that the π electron density lies over the endo or 'syn' double bond (wrt Cl) and therefore it is predicted that this alkene will be most likely to react with an electrophile.
The LUMO lies over the exo or 'anti' double bond wrt Cl. This orbital is formally electron defficient and is therefore likely to undergo electrophilic attack. It is known that dichlorocarbene is electron seeking and therefore is is predicted that the anti double bond will react when dichlorocarbene is hydrogenated.
Regioselevtive Addition to Dichlorocarbene
As the above analysis predicts, the anti double bond has been hydrogenated. A comparision between molecule 12 and the hydrogenated molecule will reveal the influence of the Cl-C bond on the vibrational frequency of the molecule. This will be done using the density functional approach.
 | 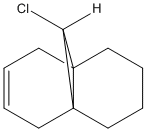 |
Both molecules 12, and the hydrogenated product were subjected to Gaussian energy minimisation using the SCAN server calculating system. Once the calculations had been run it was possible to open the files in GaussView 5 and find the C-Cl and C=C stretching vibrations for each system.
| Molecule | C=C Bond (Anti) cm-1 | C=C Bond (Syn) cm-1 | C-Cl Bond cm-1 |
| Molecule 12 | 770 | 1737 | 1757 |
| Hydrogenated Product | 780 | n/a | 1753 |
The IR spectra can be viewed below:
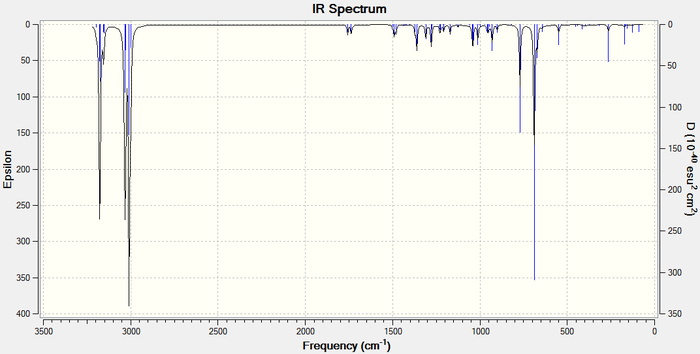
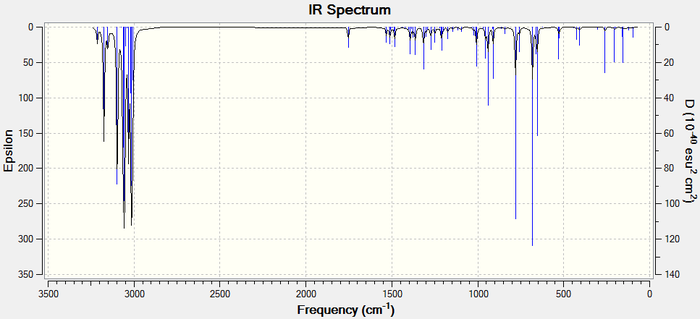
Differences betweent the IR shifts of Molecule 12 and the hydrogenated product can be seen. Most notably the 1737 anti C=C peak is only present in Molecule and not in the hydrogenated product, confirming that the double bond anti to the chlorine is most electrophilic. This can be explained using an orbital explanation. 5
A second difference between the two IR spectra is the strengthened C-Cl bond in the hydrogenated product. This suggests that the anti C=C bond was destructively interfering with the C-Cl bond in molecule 12.
Monosaccharide Chemistry: Glycosidation
The process of Glycosidation shown below leads to 2 possible anomers, the α and β anomer. For the β-anomer, the intermediate oxonium cation is attacked from the bottom face, to then allow the incoming nucleophile to replace it from the top face and the α-anomer is formed when the intermediate oxonium cation is attacked from the bottom face allowing the nucleophile to come in from the bottom face.
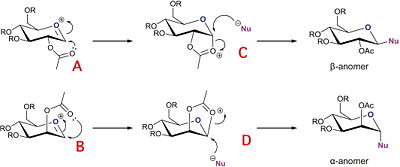
In this section, analysis of the different oxonium cation conformers A and B will reveal which is of the lower energy. The same analysis can be applied to the intermediates C and D.
An appropriate R group to represent the chemistry while keeping the computational demand minimal is CH3. OAc is the usual protecting group but given the demands of the calculations it is wise to keep the size of the molecule as small as possible without impacting the chemistry. CH3 is not too chemically different to OAc to disrupt the conformational analysis, and keeps the complexity of the calculation minimized.
The MM2 model only considers the molecular mechanics of the molecule. Given that the neighbouring group effect arises as a result of orbital interactions then the MOPAC/PM6 is a better tool for this study. This method will take into account orbital interactions and wavefuctions arising from features such as conjugation and lone pairs.
A table showing the MM2 and PM6 energies of the Monosaccharides A (acetyl group points below the plane of the oxonium cation), A* (The acetyl group points above the plane of the oxonium cation), B (acetyl group points below the plane of the oxonium cation) and B* ( (The acetyl group points above the plane of the oxonium cation)
| Molecule | Jmol | Energy MM2 | Energy PM6 |
| A | 19.51 | -90.76 | |
| A* | 27.99 | -85.88 | |
| B | 22.11 | -89.67 | |
| B* | 27.65 | -80.23 |
A similar table showing the MM2 and PM6 energies of the Intermediates C (acetyl group points below the plane of the oxonium cation), C* (The acetyl group points above the plane of the oxonium cation), D (acetyl group points below the plane of the oxonium cation) and D* ( (The acetyl group points above the plane of the oxonium cation)
C
| Molecule | Jmol | Energy MM2 | Energy PM6 |
| 34.68 | -87.37 | ||
| C* | 48.97 | -71.60 | |
| D | 40.68 | -94.19 | |
| D* | 44.87 | -70.23 |
The energy of the conformers is lower when the carbonyl group points below the plane of the oxonium cation.
Diastereospecificty is observed from the glycosidation of C to the β anomer and glycosidation of D to the α anomer.
Assigning Regioisomers in 'Click Chemistry'
Introduction
click chemistry is a chemical process that involves quickly and efficiently joining together small molecules. Click chemistry forms the basis for many reactions in nature and the term refers to a 'genre' of reactions rather than any specific reaction type. This mini project will consider a click chemistry reaction that can be catalysed by two different catalysts to give different regioisomers. Molecular modeling methods will be used to enable spectroscopic analysis of the isomers.
The 1,3-dipolar cycloaddition between an azide and an alkyne to give a 1,2,3-triazole
The reaction that will be studied in this project involves a 'click chemistry' reaction between a azide and an alkyne to give a 1,2,3-triazole.
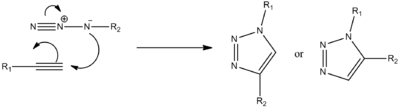
The reaction can be improved with use of a copper catalyst. (In 2002, two groups reported independently that the use of a Cu(I)-catalyst greatly speeds up the reaction.) Use of a Cu(I) catalyst however, leads to the predominant formation of isomer A. It has since been noted that use of a ruthenium (Rh(III)) catalyst leads to the predominant formation of isomer B. In this experiment the R groups will both be phenol groups. Reactions schemes for these reactions are shown below, in addition to 3D jmol representations of isomers A and B:
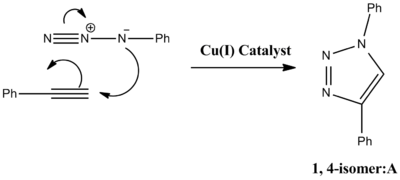
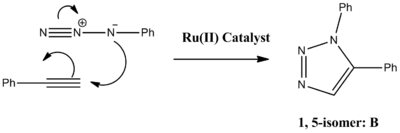
 |  |
The paper (J. Am. Chem. Soc. 2005, 127, 15998; DOI:10.1021/ja054114s ) discusses the 'Ruthenium-Catalyzed Clycloaddition of Alkynes and Organic Azides'. Using molecular modeling techniques to obtain spectroscopic data for isomers A and B, a comparison between the experimental data and the literature can be obtained.
NMR Analysis
This is done by creating a Gaussian Input File in 3DChemBio Ultra and minimising the energy using method DFT=mpw1pw91, then submitting the calculation to the shared SCAN server with instruction
"# mpw1pw91/6-31g(d,p) opt(maxcycle=25)."
A .gjf file containing the optimized geometry of the isomers can be obtained. Resubmission of the .gjf molecules (now with optimised geometry) under the instruction
"# mpw1pw91/6-31(d,p) NMR scrf(cpcm,solvent=chloroform)"
will create a .log file that can be opened in Gaussview5. In Gaussview 5, a NMR spectrum can then be viewed. The spectra are shown below:
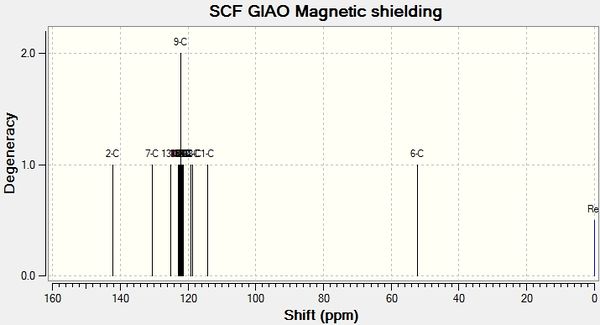
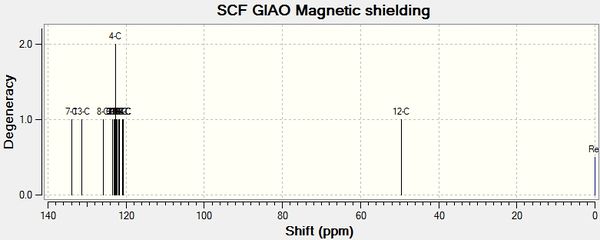
A comparison between the calculated NMR shift values and literature values can now be performed. The results have been tabulated below.
4.01| Isomer B Gaussian Shift ppm | Isomer B Literature Shift ppm 8 | Discrepancy |
| 49.51 | 51.85 | 2.34 |
| 121.95 | 126.93 | 4.98 |
| 122.34 | 127.22 | 4.88 |
| 122.46 | 128.22 | 5.76 |
| 122.75 | 128.92 | 6.17 |
| 122.89 | 129.08 | 6.19 |
| 123.14 | 129.64 | 6.5 |
| 123.48 | 133.26 | 9.78 |
| 125.86 | 133.34 | 7.48 |
| 131.65 | 135.66 | 4.01 |
| 133.94 | 138.26 | 4.32 |
| Isomer A Gaussian Shift ppm | Isomer A Literature Shift ppm | Discrepancy |
| 52.13 | 54.0 | 1.87 |
| 114.26 | 120.0 | 5.74 |
| 118.58 | 126.0 | 7.42 |
| 119.14 | 128.0 | 8.86 |
| 121.41 | 128.5 | 7.09 |
| 122.01 | 129.0 | 6.99 |
| 122.5 | 129.0 | 6.50 |
| 122.81 | 129.5 | 7.00 |
| 124.99 | 131.0 | 6.01 |
| 130.51 | 135.0 | 4.49 |
| 142.12 | 148.5 | 6.38 |
The NMR data obtained matches adequately with the literature data. The data obtained for isomer B matches best with the data presented in the paper (J. Am. Chem. Soc. 2005, 127, 15998; DOI:10.1021/ja054114s ) concuring with the fact that isomer B is formed when a Ru(II) catalyst is used.
IR Analysis
IR spectroscopy can also be helpful in analysing the two regioisomers A and B and reveal the physical differences between the two molecules.
This is done using a similar molecular modeling technique to the NMR analysis above. A Gaussian Input File in 3DChemBio Ultra and minimising the energy using method DFT=mpw1pw91, then submitting the calculation to the shared SCAN server with instruction
" # b3lyp/6-31G(d,p) opt freq"
This will create a .log file that can be opened in Gaussview5. In Gaussview 5, an IR spectrum can then be viewed. The spectra for isomers A and B are shown below:
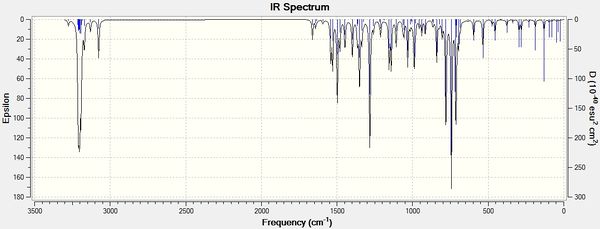
The IR data is of limited use in this analysis but does confirm that isomers A and B have different physical properties and give rise to slightly different stretching frequencies in the IR spectrum.
Optical Rotation Analysis
There are no chiral molecules in the isomers A and B and so Optical Rotation analysis would provide no useful results.
References
1. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives J. Chem. Phys. Graeme Henkelman and Hannes JónssonDOI:10.1063/1.480097
2. http://www.enc.edu/~timothy.t.wooster/courses/CH322/Lab/3-21%20The%20Diels%20Alder%20reaction.pdf}}
3. Pelayo Camps, Francesc Perez and Santiago Vdzquez, Elsevier Science Ltd, 1997, Tetrahedron, Volume 53, Issue 28, 1997, 9727-9734 DOI:10.1016/S0040-4020(97)00595-4
4. Wilhelm F. Maier, Paul Von Rague Schleye J. Am. Chem. Soc., 1981, 103 (8), pp 1891–1900DOI:10.1021/ja00398a003
5. B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. Template:DOI:10.1039/P29920000447
6. Template:Https://wiki.ch.ic.ac.uk/wiki/index.php?title=File:Anomer2.jpg
7. J. Am. Chem. Soc. 2005, 127, 15998; DOI:10.1021/ja054114s
8. Supporting Info, J. Am. Chem. Soc. 2005, 127, 15998; DOI:10.1021/ja054114s
- ↑ A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives J. Chem. Phys. Graeme Henkelman and Hannes JónssonDOI:10.1063/1.480097
- ↑ {{http://www.enc.edu/~timothy.t.wooster/courses/CH322/Lab/3-21%20The%20Diels%20Alder%20reaction.pdf}}
- ↑ |Pelayo Camps, Francesc Perez and Santiago Vdzquez, Elsevier Science Ltd, 1997, Tetrahedron, Volume 53, Issue 28, 1997, 9727-9734 DOI:10.1016/S0040-4020(97)00595-4
- ↑ Wilhelm F. Maier, Paul Von Rague Schleye J. Am. Chem. Soc., 1981, 103 (8), pp 1891–1900DOI:10.1021/ja00398a003