Rep:Mod:CPh
Module 3 (physical) of the Year 3 Computational Modelling Labs by Jan Szopinski. Please do not edit this page.
The Cope Rearrangement Tutorial
Optimizing the reactants and products
Within each of the gauche, app etc, various energy minima are possible. The experimental script contains an extensive list. Not all of them were recalculated but selected ones are presented in Table 1. The conformers will be referred to with their identifyiers from the experimental script for the sake of consistence. The energy minima were confrmed by the absence of imaginary frequencies.
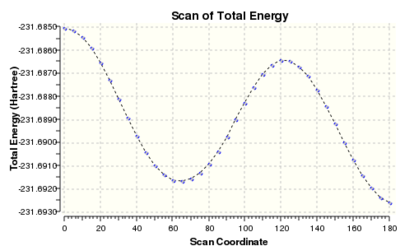
Scan
The scan profile of the central dihedral (C2-C3-C4-C5) of a butane molecule is well-known. Staggered conformations are energy minima, while eclipsed conformations can be regarded as transition states between them. It is also known that antiperiplanar structure is slightly (0.9 kcal/mol) lower in energy than gauche conformations. To find the influence of unsaturation on the both ends of hexadiene molecule, a relaxed scan of the dihedral angle around the central C-C bond was performed. The starting structure had an s-cis conformation with carbon atoms contained in one plane. The calculation was conducted at HF/3-21G and consisted of 36 steps of 5 degrees, encompassing a half of the full angle, which is sufficient due to the symmetry of the molecule. The scan profile is shown on the right. The minimum just above 60 degrees corresponds to a gauche conformation and the minimum at 180 corresponds to antiperiplanar conformation. The calculation suggests that the gauche conformation is higher in energy than the app by 0.60 kcal/mol.
Anti
Two app minima were found. The first one was found in an optimization performed on the structure used in the scan but cleaned by GV. The optimized structure and energy corresponded to the anti1 entry in the experimental script. To find another minimum of the app conformation, the C1-C2-C3-C4 dihedral angle in the cleaned structure was changed from 150 to -150 degrees. This optimization yielded a different local minimum. Their energies are included in the table. The energy difference was...
Gauche
Similarly, to give a more varied and representative view of the structures, two minima were found. The initial structure was first one was found by changing the central dihedral from 180 to 60. Optimizing such structure yielded gauche2, and cleaning before optimization gauche4. The lowest energy conformer, as per experimental script, gauche3 was not found. To locate it, the gauche 2 from ca -123 to 150 degrees. The global minimum was then successful reached in the optimization.
Summary of structures
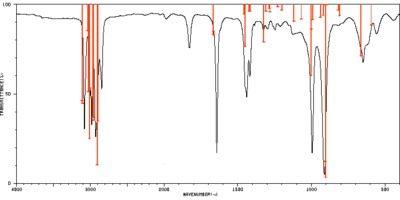
Frequency analysis and thermochemistry of gauche3 conformer
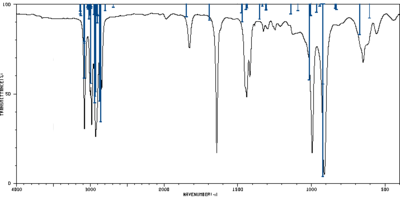
The frequency calculation was performed at B3LYP/6-31G* level of theory. The relevant scaling factors are 0.9613 for frequencies and 0.9804 for thermochemical values. [1] A frequency calculation with anharmonic corrections was also conducted at the same level of theory. The table below shows all calculated frequencies with assigned vibrations. The assignment was based on visual assessment of the animation in GaussView 5, however a more accurate and formal assignment could be conducted using the Total Energy Distribution method.[2] The experimental spectrum from Spectral Database of Organic Compounds[3] was compared with both theoretical spectra in the table below. All theoretical intensities were normalized to the peak of maximum intensity treated as 100%.
|
Experimental |
B3LYP/6-31G* |
||||||
|
SDBS |
Harmonic (scaled by 0.9613) |
Anharmonic |
|||||
|
Wavenumber |
100% - T |
Wavenumber |
Intensity |
Assignment |
Wavenumber |
Intensity |
Assignment |
|
557 |
21 |
594 |
12 |
C=CH2 twisting |
606 |
8 |
← |
|
649 |
34 |
663 |
30 |
673 |
18 |
← |
|
|
|
|
809 |
7 |
C=CH2 and CH2 rocking |
|
|
|
|
913 |
96 |
903 |
100 |
C=CH2 wagging |
922 |
46 |
← |
|
904 |
91 |
923 |
100 |
← |
|||
|
919 |
6 |
C=CH2 and CH2 rocking |
|
|
|
||
|
939 |
8 |
|
|
|
|||
|
|
|
|
|
|
966 |
5 |
C=CH2 rocking and C=CH bending |
|
994 |
84 |
991 |
9 |
C=CH2 twisting and CH2 wagging |
1005 |
7 |
C=CH torsion |
|
1001 |
14 |
C=CH torsion |
1008 |
7 |
C=CH2 twisting and CH2 wagging |
||
|
1003 |
41 |
1015 |
44 |
← |
|||
|
|
|
1069 |
8 |
C=CH torsion+bending and CH2 twisting |
1092 |
4 |
← |
|
|
|
1119 |
10 |
C=CH bending and C=CH2 rocking |
1140 |
6 |
← |
|
1207 |
16 |
|
|
|
|
|
|
|
1213 |
16 |
|
|
|
|
|
|
|
1247 |
19 |
|
|
|
|
|
|
|
1257 |
16 |
|
|
|
|
|
|
|
1298 |
16 |
1281 |
4 |
C=CH bending and C=CH2 scissoring |
1310 |
4 |
C=CH bend |
|
1308 |
4 |
CH2 twisting |
|
|
|
||
|
1327 |
19 |
1324 |
22 |
CH2 wagging |
1352 |
9 |
CH2 wagging |
|
1384 |
16 |
|
|
|
|
|
|
|
1418 |
43 |
1416 |
4 |
C=CH2 scissoring |
1469 |
10 |
CH2 scissoring |
|
1422 |
4 |
|
|
|
|||
|
1440 |
54 |
1451 |
24 |
CH2 scissoring |
1475 |
5 |
← |
|
1457 |
6 |
|
|
|
|||
|
1643 |
84 |
1665 |
18 |
C=C stretch |
1692 |
10 |
← |
|
1666 |
16 |
|
|
|
|||
|
1828 |
28 |
|
|
|
1849 |
7 |
C=CH2 wagging overtone |
|
|
|
|
1851 |
7 |
|||
|
|
|
|
|
|
2799 |
4 |
CH2 scissoring overtone |
|
2847 |
50 |
2895 |
67 |
C-H2 symmetric stretch |
2860 |
68 |
← |
|
2906 |
93 |
2872 |
56 |
C-H2 symmetric stretch |
|||
|
|
|
|
|
|
2902 |
7 |
Combination band of 1475 and CH2 scissoring |
|
|
|
|
|
|
2907 |
11 |
|
|
|
|
|
|
|
2911 |
6 |
Combination band of 1475 and C=CH2 scissoring |
|
|
|
|
|
|
2914 |
5 |
|
|
2928 |
74 |
2954 |
67 |
C-H2 antisymmetric stretch |
2930 |
51 |
← |
|
2963 |
65 |
2936 |
53 |
C-H2 antisymmetric stretch |
|||
|
|
|
|
2942 |
57 |
C=C-H stretch |
||
|
|
|
|
2952 |
6 |
Combination of CH2 scissoring 1469 and 1475 |
||
|
2981 |
69 |
3010 |
78 |
C=C-H stretch |
2994 |
46 |
C=C-H2 symmetric and C=C-H stretch |
|
3001 |
53 |
3028 |
50 |
C=C-H2 symmetric and C=C-H stretch |
3005 |
6 |
|
|
3035 |
15 |
3017 |
8 |
||||
|
3037 |
15 |
|
|
|
|||
|
3081 |
71 |
3107 |
57 |
C=C-H2 antisymmetric stretch |
3082 |
23 |
C=C-H2 antisymmetric stretch |
|
3110 |
56 |
3083 |
43 |
||||
|
|
|
|
3086 |
21 |
CH2 scissoring overtone |
||
|
|
|
|
|
|
3133 |
7 |
Combination of C=CH2 scissoring and C=C stretch |
|
|
|
|
|
|
3136 |
5 |
Combination of 1692 and C=CH2 scissoring |
|
|
|
|
|
|
3138 |
4 |
Combination of C=CH2 scissoring and C=C stretch |
Selected peaks of normalized intensities above 4% were assigned to their experimental counterparts to compare the harmonic and anharmonic calculations. Standard deviation for both methods in two frequency regions were calculated and the results are summarized in the table below. While the assignment was to some degree arbitrary, it is clear that harmonic frequencies are better than anharmonic for low wavenumbers and the situation is opposite for the high wavenumber region. This agrees with the fact that high frequency vibrations contain a high degree of anharmonictiy, which has to be accounted for.
| Experimental | Harmonic | Anharmonic | ||
| Wavenumber / cm-1 |
Wavenumber / cm-1 |
Difference squared / cm-2 |
Wavenumber / cm-1 |
Difference squared / cm-2 |
| 557 | 594 | 1380 | 606 | 2392 |
| 649 | 663 | 192 | 673 | 585 |
| 913 | 905 | 63 | 923 | 97 |
| 994 | 1001 | 46 | 1013 | 376 |
| 1298 | 1294 | 16 | 1310 | 135 |
| 1327 | 1324 | 9 | 1352 | 638 |
| 1418 | 1419 | 1 | 1469 | 2648 |
| 1440 | 1452 | 142 | 1475 | 1199 |
| 1643 | 1665 | 499 | 1692 | 2391 |
| Standard deviation of low wavenumbers | 16 cm-1 | 34 cm-1 | ||
| 2847 | 2902 | 2975 | 2866 | 344 |
| 2928 | 2958 | 921 | 2937 | 75 |
| 2981 | 3010 | 870 | 2994 | 171 |
| 3001 | 3031 | 892 | 3012 | 115 |
| 3081 | 3108 | 746 | 3083 | 5 |
| Standard deviation of high wavenumbers | 36 cm-1 | 12 cm-1 | ||
| Overall standard deviation | 25 cm-1 | 28 cm-1 | ||
Optimizing the transition structures
The chair transition state structure has been found by requesting optimization from a sensible guess structure, which was obtained by optimizing with frozen reaction coordinate. The transition state was confirmed by one imaginary frequency obtained in frequency vibration at the same level of theory. An IRC calculation proved that the reaction leads to a gauche2 conformation.
A boat transition structure was obtained from the QST2 algorithm implemented in Gaussian. An imaginary frequency confirmed that it was the structure sought for. An IRC calculation showed that the reaction leads to a conformer, which itself is a transition structure with respect to central dihedral bond torsion.
The Diels Alder Cycloaddition
Reaction of Butadiene with Ethene
Molecular orbital analysis of reactants
The Highest Occupied and Lowest Unoccupied Molecular orbitals of ethene and butadiene are shown in the table below. The symmetry of orbitals with respect to the central plane can be easily seen from the figures. HOMO of ethene and LUMO of butadiene are symmetric, while LUMO of butadiene and HOMO of ethene are antisymmetric. This match allows them to interact in a cycloaddition reaction.
| HOMO | LUMO | |
|---|---|---|
| ethene |  |

|
| butadiene | 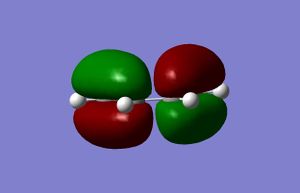 |

|
Energies and location of transition state
A guess for the structure of transition was made by making slight changes to the reactants and arranging them in a sensible manner. An optimization to transition state was requested using the AM1 semi-empirical method and a frequency calculation at the same level of theory showed one imaginary frequency, which is presented on the central figure and agrees with the expected vibration. An IRC calculation in both direction confirms that the transition state is between reactants (figure on the left) and the cycloaddition product (right). A plot of the IRC calculation allows to estimate the reaction energy barrier. A more accurate result can be obtained from energy values in the table below.
The bond length of the both sigma bonds being formed in the transition structure was calculated to be 2.119 Å. As this bond length was almost identical in the transition state, it can be concluded that this step was synchronous, which agrees with the simultaneous displacements in the imaginary frequency visualisation. At the same time, the central butadiene bond has shortened from 1.449 Å to 1.397 Å.
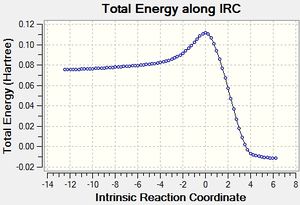
The lowest real frequency (147 cm-1) corresponds to a rotation of the molecules with respect to each other. As a real vibration is expected to, it does not seem to lead to any bond breaking.
Molecular orbitals of transition state
The HOMO and LUMO of the transition state structure are shown on Figures X and Y respectively. It can be seen from the figures, that the HOMO is antisymmetric and LUMO antisymmetric with respect to the central plane. The symmetry considerations allow to conclude the following mixing relations:
antisymmetric: HOMOTS = HOMObutadiene + LUMOethene
symmetric: LUMOTS = LUMObutadiene + HOMOethene
Orbital |
Orbital |
Reaction of 1,3-cyclohexadiene with maleic anhydride
Molecular orbital analysis of reactants
The symmetry with respect to the central plane has been noted in the table below. Similarly to the reaction of butadiene and ethene, this reaction is allowed as there is a symmetry match between the HOMO of maleic anhydride with LUMO of 1,3-cyclohexadiene, as well as a match between HOMO of the latter with the LUMO of the former.
| HOMO | LUMO | |
|---|---|---|
| maleic anhydride | 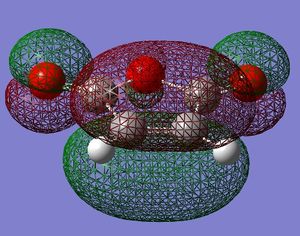 symmetric |
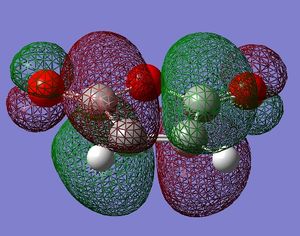 antisymmetric |
| 1,3-cyclohexadiene | 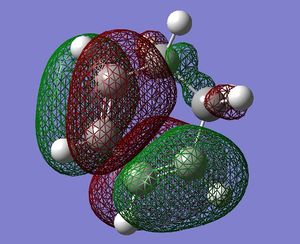 antisymmetric |
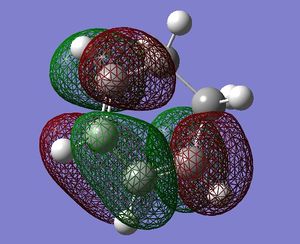 symmetric |
Energies and location of transition state
The table below summarizes the energies of reactant, product and transition state molecules. To obtain a higher level of accuracy, a B3LYP/6-31G* level of theory was employed for stationary point structures. The results show that the endo form is lower in energy by about 1.6 kcal/mol.
| Molecule | Energy (AM1) / Hartree |
Energy (B3LYP/6-31G*) / Hartree |
|---|---|---|
| Maleic anhydride | -0.12182 | -379.28954 |
| 1,3-cyclohexadiene | 0.02771 | -233.41894 |
| endo TS | -0.05151 | -612.68340 |
| endo product | -0.16017 | -612.75829 |
| exo TS | -0.05042 | -612.67931 |
| exo product | -0.15991 | -612.75579 |
Molecular orbitals of transition state
The interactive figures in the table present the HOMO and LUMO orbitals of the endo and exo transition states. All orbitals were antisymmetric with respect to the central plane
| approach | HOMO | LUMO | ||||||
|---|---|---|---|---|---|---|---|---|
| endo |
|
| ||||||
| exo |
|
|
Abbreviations
app = antiperiplanar
TS = transition state
References
- ↑ Exploring Chemistry With Electronic Structure Methods, James B. Foreman, Gaussian, 2nd edition, 1996
- ↑ A.E. Yavuz, S. Haman Bayarı, N. Kazancı, Structural and vibrational study of maprotiline, Journal of Molecular Structure, 924-926,2009,313-321
- ↑ http://sdbs.riodb.aist.go.jp/sdbs/cgi-bin/cre_index.cgi Accessed: 28/10/2013