Rep:Mod:CHEM126825124225121800
Part 1A - Confirmational Analysis using Molecular Mechanics
The Hydrogenation of Cyclopentadiene Dimer
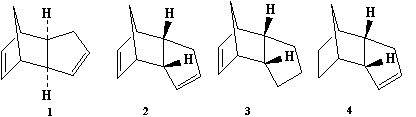
Compounds 1 and 2
Cyclopentadiene readily undergoes a Diels Alder cycloaddition to form a dimeric product. Dimerisation is stereospecific, and results in the endo product (1) over the exo product (2). A series of optimisations were conducted in Avogadro using the parameters below to obtain the most stable conformations and energies for comparison.
- Force Field: MMFF94s
- Algorithm: Conjugate Gradients
| Compound | TOTAL ENERGY (kcal/mol) | CML |
|---|---|---|
| 1 | 55.37610 | link |
| 2 | 58.20369 | link |
Calculations showed the exo isomer (1) to be more stable by 2.82759 kcal/mol than the endo isomer (2). The experimental observations however show 2 to be formed preferentially over 1 despite 2 being higher in energy.
Chemical reactions are driven by having negative free energy changes, and the larger that change the more thermodynamically stable the product is. However, the activation energy (or barrier) over which the reactants must overcome in order to be converted into products is also important. It can therefore be seen that the dimerisation of cyclopentadiene is controlled by kinetic factors relating to the transition state (i.e. the transition state energy for the formation of 2 is lower than 1 and thus it is formed faster).
Compounds 3 and 4
Compounds 3 and 4 were analysed in a similar fashion to above.
- Force Field: MMFF94s
- Algorithm: Conjugate Gradients
| Compound | Bond Stretching Energy (kcal/mol) | Angle Bending Energy (kcal/mol) | Tortional Stretch Bending Energy (kcal/mol) | Tortional Energy (kcal/mol) | Out-of-plane Bending Energy (kcal/mol) | Van der Walls Energy (kcal/mol) | Electrostatic Energy (kcal/mol) | TOTAL ENERGY (kcal/mol) | CML |
|---|---|---|---|---|---|---|---|---|---|
| 3 | 3.31272 | 31.98784 | -2.10535 | -1.51908 | 0.01282 | 13.63753 | 5.11947 | 50.44595 | link |
| 4 | 2.82708 | 24.68137 | -1.65942 | -0.39444 | 0.00026 | 10.65877 | 5.14692 | 41.26055 | link |
| Difference | 0.48564 | 7.30647 | -0.44593 | -1.12464 | 0.01256 | -2.97876 | -0.02745 | 9.1854 |
The biggest difference to the stability of 3 and 4 can be seen in the angle bending energy (4 shows an angle bending energy value 7.30647 kcal/mol less than that of 3). Despite this, many of the values reported in the differences row are actually negative indicating that the value for 4 is greater than 3. Overall however, 4 is lowest in energy.
[[
Image:Taxol molecules.gif|center|400px]]
A variety of conformations of the 6 member ring in Taxol were observed. ChemBio 3D was used to optimise the geometry by a series of energy optimisations and manual manipulation of atoms. Both chair and twist boat conformations were found, with the chairs usually being lower in energy. Interestingly, for Compound 10, one of the chairs found is actually higher in energy than both of the twist boats.
- Force Field: MM2
| Compound 9 | Compound 10 | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
The data obtained shows the most stable antropisomer to be isomer 10, with the 6 membered ring in one of the 2 chair conformations. This conformation has an energy of 42.6828 kcal/mol. This atropisomer is thus likely to react in carbonyl addition reactions.
A series of compounds known as hyperstable alkenes are known in literature[1]. These compounds are less strained than their saturated hydrocarbon derivatives and thus shown unusual stability to reaction. The energy difference between a hydrocarbon and its olefin derivative is known as the olefin strain energy (OS). By definition, hyperstable alkenes have negative OS values. Bridgehead olefins that have twisted geometries often show hyperstable alkene behaviour. Thus the alkene functionality in isomers 8 and 9 is likely to show reduced reactivity as they contain this motif.
Taxol has indeed been proposed in literature[2] to exibit hyperstable alkene behaviour when annalysed at the MM2 level.
Part 1B: Spectroscopic Simulation using Quantum Mechanics
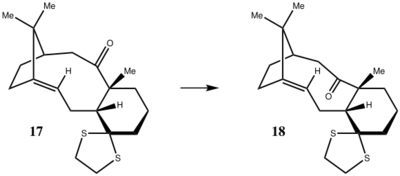
Both Compounds 17 and 18 were modeled with respect to energy, but the NMR data is only reported for Compound 18. The geometry was optimized in ChemBio3D using an MM2 force field.
Geometries were optimised at the density functional level using B3LYP and a 6-31G(d,p) basis set. An EmpiricalDispersion=GD3 factor was also applied.
| Compound | 17 | 18 |
|---|---|---|
| Geometry |  |
 |
| jmol | ||
| MM2 Optimised Energy | 63.9623 kcal/mol | 64.3641 kcal/mol |
| Free Energy (Sum of electronic and thermal free energies) |
-1651.393028 Hartrees | -1651.394012 Hartrees |
| DOI | DOI:10042/27067 | DOI:10042/27068 |
The data computed for the compounds by quantum mechanics shows Compound 18 to be slightly more stable than 17.
1H NMR
| Computed 1H Spectrum | Computed 1H Data (Benzene) |
Experimental 1H Data[3] (300 MHz, C6D6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
|
|
13C NMR
| Computed 13C | Computed 13C Data (Benzene) |
Experimental 13C Data[3] (76 MHz, C6D6) |
Difference | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
|
|
|
A comparison of the NMR data to literature.
The 13C NMR data computed as a whole agrees well to literature. Although the resonance reported in literature at 74.61 ppm differs largely to that computed of 92.84 ppm, the remaining data is in good agreement.
On the otherhand, the 1H NMR data shows significant differences to that computed. Many of the multiplets reported in literature that integrated to a number of hydrogens are obtained as seperate resonaces in the computed spectrum. Some peaks are in approximate agreement to that found experimentally. For example, the multiplet reported in literature at 5.21 ppm occurs at 5.97 ppm in the calculated spectrum.
PART 2: Analysis of the properties of the synthesised alkene epoxides
Crystal structures of The Shi and Jacobsen catalysts
|
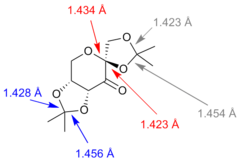 |
Bond lengths for the the anomeric centre contained within the 6 member ring in the Shi catalysts were found. The O-C-O bond lengths were 1.434 Å and 1.423 Å. Further to this, other C-O bond distances were observed and are labelled in the figure above.
The anomeric effect is usually observed in cyclohexane rings that contain a heteroatom. It is defined by the fact that substituents on carbons next to the atom have a preference for an axial orientations rather than the expected equatorial which would minimise 1,3-diaxial repulsions. A lone pair on the heteroatom is able to overlap with an antiperiplanar antibonding C-X orbital from the substituent in a favourable, stabilising interaction. This shortens the C-O bond between the heteroatom in the ring and the subsituent as it now has more double bond character. Conversely, the C-X bond is elongated as electron density is placed into an antibonding orbital.
|
The closest approach between hydrogens atoms on the adjacent t-butyl groups in the Jacobsen catalyst were measured as 2.421 Å. This gives a carbon - carbon closest approach distance between these groups of 3.696 Å. It should be noted that these are obtained from the crystal structure. It can be inferred that when the molecule 'froze' to crystallise, it would do so in its lowest energy orientation with these t-butyl groups as far apart as possible.
The Van der Walls radius of hydrogen has been determined as 1.20 Å.[4] Therefore, 2x the VdW radius gives a value that is just less (2.40 Å) than the measured closest approach of 2.421 Å. Therefore, in the crystal state (inferred as the lowest energy as above), there is marginal steric interactions between the t-butyl groups.
Calculated NMR properties of epoxides from Styrene and Trans-stilbene
An epoxide from Styrene - (R)-2-phenyloxirane
| 1H NMR | 13C NMR | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
 | ||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||
| DOI:10042/27069 | |||||||||||||||||||||||||||||
Coupling Constants
Coupling constants were obtained by running a quantum mechanical calculation. DOI:10042/27340
Total nuclear spin-spin coupling J (Hz):
1 2 3 4 5
1 0.000000D+00
2 0.574369D+02 0.000000D+00
3 -0.151392D+01 0.573242D+02 0.000000D+00
4 0.982212D+01 -0.207013D+01 0.567495D+02 0.000000D+00
5 -0.121803D+01 0.939092D+01 -0.134967D+01 0.595917D+02 0.000000D+00
6 0.577241D+02 -0.211450D+01 0.968070D+01 -0.333490D+00 0.618056D+02
7 0.440000D+01 -0.177196D+01 0.502033D+01 0.368208D+01 0.556341D+02
8 -0.878747D+00 0.154325D+01 -0.157807D+00 0.659424D+01 -0.492838D+00
9 0.167609D+03 0.208042D+01 0.806257D+01 -0.138845D+01 0.752792D+01
10 0.223460D+01 0.166009D+03 0.200431D+01 0.778470D+01 -0.159630D+01
11 0.799587D+01 0.185755D+01 0.167057D+03 0.240538D+01 0.745455D+01
12 -0.106381D+01 0.760809D+01 0.116350D+01 0.165818D+03 0.179055D+01
13 0.552225D+00 0.759010D+01 -0.122778D+01 0.731163D+01 0.149401D+01
14 0.744998D+00 0.222256D+00 -0.130634D+00 0.547577D+01 0.408296D+01
15 0.360732D+00 -0.476003D+00 0.751845D+00 -0.303430D+00 0.120264D+02
16 0.426009D+00 -0.427786D+00 0.353852D+00 -0.731781D+00 0.661508D+01
6 7 8 9 10
6 0.000000D+00
7 0.233682D+01 0.000000D+00
8 0.520562D+01 0.729416D+02 0.000000D+00
9 0.265339D+01 0.627324D+00 0.734431D-01 0.000000D+00
10 0.798593D+01 0.888566D+00 -0.572611D+00 0.852548D+01 0.000000D+00
11 -0.143813D+01 0.155293D+00 0.342169D+00 0.101402D+01 0.866338D+01
12 0.655886D+01 0.676474D+01 -0.251467D+00 0.367007D+00 0.783142D+00
13 0.164157D+03 0.406130D+01 -0.463702D+00 0.892216D+01 0.735697D+00
14 0.586432D+01 0.162126D+03 -0.416555D+00 0.504130D+00 -0.385559D+00
15 -0.517922D+00 -0.512997D-01 0.166786D+03 -0.250424D+00 0.314431D+00
16 -0.530606D+00 -0.216723D+01 0.162867D+03 -0.115438D+00 0.202286D+00
11 12 13 14 15
11 0.000000D+00
12 0.860881D+01 0.000000D+00
13 0.469567D+00 0.172501D+01 0.000000D+00
14 -0.157690D-01 -0.152639D+00 -0.838173D+00 0.000000D+00
15 -0.129756D+00 0.304893D+00 -0.257744D-01 0.126795D+02 0.000000D+00
16 -0.314741D+00 0.232354D+00 0.439559D+00 0.181908D+02 0.696814D+00
16
16 0.000000D+00
An epoxide from Trans-stilbene - trans–1,2–diphenylethylene oxide [S,S]
| 1H NMR | 13C NMR | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
 | ||||||||||||||||||||||||
|
| ||||||||||||||||||||||||
| DOI:10042/27070 | |||||||||||||||||||||||||
Coupling Constants
Coupling constants were obtained by running a quantum mechanical calculation. DOI:10042/27339
Total nuclear spin-spin coupling J (Hz):
1 2 3 4 5
1 0.000000D+00
2 0.603403D+02 0.000000D+00
3 -0.115501D+01 0.611460D+02 0.000000D+00
4 0.931627D+01 -0.269161D+00 0.613415D+02 0.000000D+00
5 -0.110762D+01 0.102534D+02 0.100420D+01 0.633677D+02 0.000000D+00
6 0.606625D+02 -0.124262D+00 0.105389D+02 -0.193319D+00 0.622889D+02
7 -0.589146D+00 0.548106D+00 -0.667743D+00 0.509412D+01 -0.555655D+00
8 0.569435D+00 -0.312655D+00 0.600199D+00 -0.667935D+00 0.495269D+00
9 -0.335600D+00 0.236087D+00 -0.312703D+00 0.548251D+00 -0.343116D+00
10 0.544321D+00 -0.335683D+00 0.569360D+00 -0.589211D+00 0.551924D+00
11 -0.318380D+00 0.219877D+00 -0.319221D+00 0.427289D+00 -0.232671D+00
12 0.551844D+00 -0.343096D+00 0.495230D+00 -0.555803D+00 0.789643D+00
13 -0.213892D+01 0.561821D+01 0.223063D+01 0.695447D+02 0.121665D+01
14 0.185169D+01 -0.134769D+00 0.737420D+01 0.455281D+00 0.730224D+01
15 0.156421D+03 0.274451D+00 0.816330D+01 -0.136208D+01 0.844501D+01
16 -0.718645D-01 0.156351D+03 0.109598D+01 0.717877D+01 -0.125338D+01
17 0.831908D+01 -0.129332D+01 0.153624D+03 0.230372D+01 0.721360D+01
18 0.823540D+01 -0.101648D+01 0.834429D+01 0.216952D+01 0.151134D+03
19 0.199188D+00 0.874702D+01 -0.123811D+01 0.715544D+01 0.142082D+01
20 -0.212057D+00 0.133671D+00 -0.225389D+00 0.309365D+00 -0.197067D+00
21 0.234419D-01 -0.757270D-01 0.464971D-01 -0.822724D-01 0.378130D-01
22 -0.204864D+00 0.149832D+00 -0.207106D+00 0.347199D+00 -0.203496D+00
23 0.216012D-01 -0.671917D-01 0.447700D-01 -0.114347D+00 0.303178D-01
24 -0.217186D+00 0.159494D+00 -0.888279D-01 0.282248D+00 -0.105807D+00
25 0.342470D+00 0.164536D+00 0.989268D+01 0.103757D+01 0.711774D+01
26 -0.459746D+00 0.381101D+00 -0.758563D+00 0.598834D+01 -0.538683D+00
6 7 8 9 10
6 0.000000D+00
7 0.427356D+00 0.000000D+00
8 -0.319286D+00 0.613456D+02 0.000000D+00
9 0.219865D+00 -0.269613D+00 0.611395D+02 0.000000D+00
10 -0.318541D+00 0.931577D+01 -0.115521D+01 0.603474D+02 0.000000D+00
11 0.226513D+00 -0.193500D+00 0.105372D+02 -0.124444D+00 0.606585D+02
12 -0.232615D+00 0.633494D+02 0.100325D+01 0.102548D+02 -0.110793D+01
13 0.471414D+01 0.454811D+00 0.737569D+01 -0.135127D+00 0.185182D+01
14 -0.767476D+00 0.695511D+02 0.223021D+01 0.561866D+01 -0.213883D+01
15 0.571986D+00 0.347200D+00 -0.207197D+00 0.149869D+00 -0.204807D+00
16 0.855148D+01 -0.821676D-01 0.463905D-01 -0.756895D-01 0.235469D-01
17 -0.780448D+00 0.309282D+00 -0.225429D+00 0.133700D+00 -0.212037D+00
18 -0.236206D+01 0.282101D+00 -0.887614D-01 0.159482D+00 -0.217422D+00
19 0.156801D+03 -0.114237D+00 0.446540D-01 -0.671630D-01 0.217569D-01
20 0.155776D+00 0.230579D+01 0.153631D+03 -0.129332D+01 0.831776D+01
21 -0.691329D-01 0.717778D+01 0.109593D+01 0.156350D+03 -0.724360D-01
22 0.146631D+00 -0.136214D+01 0.816412D+01 0.276852D+00 0.156418D+03
23 -0.756374D-01 0.715644D+01 -0.123851D+01 0.874615D+01 0.199532D+00
24 0.133966D+00 0.216708D+01 0.834233D+01 -0.101621D+01 0.823611D+01
25 0.651660D+00 0.598861D+01 -0.759011D+00 0.381346D+00 -0.459659D+00
26 0.449923D+00 0.103837D+01 0.989358D+01 0.164404D+00 0.342615D+00
11 12 13 14 15
11 0.000000D+00
12 0.622973D+02 0.000000D+00
13 -0.767042D+00 0.730272D+01 0.000000D+00
14 0.471203D+01 0.121674D+01 0.705725D+02 0.000000D+00
15 0.146526D+00 -0.203565D+00 0.949545D+00 -0.708766D+00 0.000000D+00
16 -0.692341D-01 0.377937D-01 -0.104754D+00 0.324524D+00 0.117587D+02
17 0.155582D+00 -0.197159D+00 0.936101D+01 -0.205850D+00 0.876609D+00
18 0.134076D+00 -0.105698D+00 0.500211D+01 -0.409109D+00 0.789382D+00
19 -0.757643D-01 0.302569D-01 0.614638D+00 0.111608D+00 0.115700D+02
20 -0.780605D+00 0.721394D+01 -0.206283D+00 0.936035D+01 0.615458D-01
21 0.855310D+01 -0.125333D+01 0.324518D+00 -0.104615D+00 -0.123831D+00
22 0.569703D+00 0.844474D+01 -0.708867D+00 0.949479D+00 0.115369D+00
23 0.156796D+03 0.142083D+01 0.111617D+00 0.614547D+00 -0.122036D+00
24 -0.235732D+01 0.151136D+03 -0.409157D+00 0.499979D+01 0.406319D-01
25 0.449441D+00 -0.538991D+00 0.144797D+03 -0.316766D+00 -0.498488D+00
26 0.651764D+00 0.711871D+01 -0.317531D+00 0.144795D+03 0.318097D+00
16 17 18 19 20
16 0.000000D+00
17 0.120152D+02 0.000000D+00
18 0.221080D+00 0.248858D+01 0.000000D+00
19 0.122893D+01 0.103421D+00 0.125247D+02 0.000000D+00
20 -0.137715D+00 0.662458D-01 0.315729D+00 -0.491621D-01 0.000000D+00
21 -0.495353D-01 -0.137705D+00 -0.729010D-01 -0.256857D-01 0.120133D+02
22 -0.123828D+00 0.615172D-01 0.406273D-01 -0.122040D+00 0.876366D+00
23 -0.256932D-01 -0.491399D-01 -0.136702D+00 -0.535066D-01 0.103554D+00
24 -0.728783D-01 0.315705D+00 0.982935D-01 -0.136697D+00 0.248842D+01
25 0.129070D+00 -0.405769D+00 -0.119036D+01 0.725008D+00 0.344295D+00
26 -0.319480D+00 0.344068D+00 0.318672D+00 -0.111296D+00 -0.405924D+00
21 22 23 24 25
21 0.000000D+00
22 0.117591D+02 0.000000D+00
23 0.122884D+01 0.115700D+02 0.000000D+00
24 0.221036D+00 0.789263D+00 0.125243D+02 0.000000D+00
25 -0.319525D+00 0.318122D+00 -0.111334D+00 0.318711D+00 0.000000D+00
26 0.129124D+00 -0.498484D+00 0.725226D+00 -0.119044D+01 0.182685D+02
26
26 0.000000D+00
The reported literature for optical rotations
Reaxys was searched using specific stereoisomers. All values reported in Reaxys at 589 nm in the correct solvent were analysed and then averaged. In the case of (R)-2-phenyloxirane, the sign of the value reported was variable. Details of the calculation are available by following the links in the table below. Only values reported in chloroform were used to determine the literature average as this was the solvent used in the computations.
| Epoxide | Stereochemistry | Average Literature Optical Rotation at 589 nm |
|---|---|---|
| 2-phenyloxirane | [R] | - 22.42 o Link to data summary |
| trans–1,2–diphenylethylene oxide | [S,S] | - 227.10 o Link to data summary |
The calculated chiroptical properties of the product
| Epoxide | Stereochemistry | OR | ECD | VCD |
|---|---|---|---|---|
| 2-phenyloxirane | [R] | -30.37 o |  |
 |
| DOI:10042/27088 | DOI:10042/27091 | DOI:10042/27090 | ||
| trans–1,2–diphenylethylene oxide | [S,S] | -298.12 o |  |
 |
| DOI:10042/27096 | DOI:10042/27097 | DOI:10042/27098 |
The calculated optical rotation for (R,R)-2-phenyloxirane agrees very well to the average value obtained from a literature search. That of trans–1,2–diphenylethylene oxide however does not agree as favorably.
Using the calculated properties of the transition state for the reaction
Using the following equations, and free energies reported for the transition states by quantum mechanical calculations, the enantiomeric excess for the products of the epoxidation of trans-β-methyl styrene using two different catalysts are shown below.
Δ
Shi Catalyst Epoxidation of trans-β-methyl styrene
The table below reports the energy of the lowest transition state provided in the experimental script, for each of the possible stereoisomeric products with the specific catalyst used.
| Transition States | Stereochemistry | Energy TS | |||
|---|---|---|---|---|---|
| Shi Epoxidation of trans-β-methyl styrene |
R,R | -1343.032443 | |||
| S,S | -1343.024742 | ||||
| ΔE (Hartrees) | -0.007701 | ||||
| ΔE (kJ/mol) | -20.21897704 | ||||
| K | 3500.996818 | ||||
| Enantomeric Excess | 99.943 % | ||||
This calculated enantiomeric excess predicts an excess of the (R,R) diastereoisomer to be formed.
A literature search[5] yielded results from previous experimental work that supported these conclusions - i.e. that the (R,R) enantiomer was made selectively.
Jacobsen Catalyst Epoxidation of trans-β-methyl styrene
| Transition States | Stereochemistry | Energy TS | |||
|---|---|---|---|---|---|
| Jacobsen Epoxidation of trans-β-methyl styrene |
R,R | -3383.254344 | |||
| S,S | -3383.262481 | ||||
| ΔE (Hartrees) | 0.008137 | ||||
| ΔE (kJ/mol) | 21.3636951 | ||||
| K | 0.000179949 | ||||
| Enantiomeric Excess | 99.996 % | ||||
The Jacobsen epoxidation[6] is known to prefer cis alkene subjects to enantiopure epoxidations. Although examples of the epoxidation of cis-β-methyl styrene could be found in literature, the equivalent for trans-β-methyl styrene were not found.
Investigating the non-covalent interactions in the active-site of the reaction transition state
- The TS for (R,R)-trans-β-methyl styrene was investigated using the Shi catalyst.
- The following parameters were used:
- Type = Total Density
- Grid = Fine
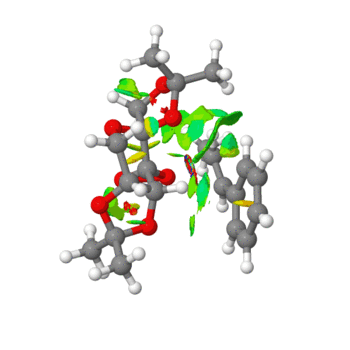 |
|
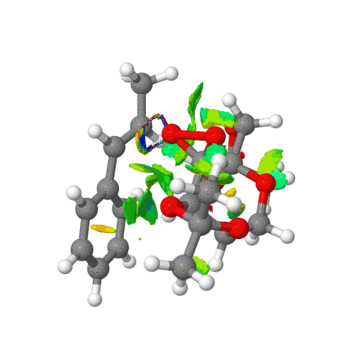 |
In the above images, a number of interactions can be observed. Those in green are weakly attractive, in blue strongly attractive, in yellow are weekly repulsive and in in red are strongly repulsive. A new bond can be seen to be forming in the right hand image between the C-C of the alkene in the starting material and an oxygen atom on the Shi catalyst. Extensive green areas are observed on the NCI analysis which indicate substantial non covalent interactions occuring in the active site of the reaction transition state.
Investigating the Electronic topology (QTAIM) in the active-site of the reaction transition state
- The TS for (R,R)-trans-β-methyl styrene was investigated.
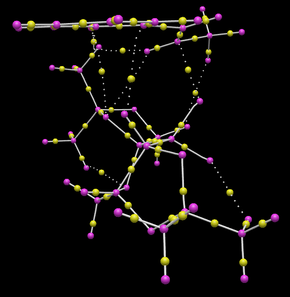 |
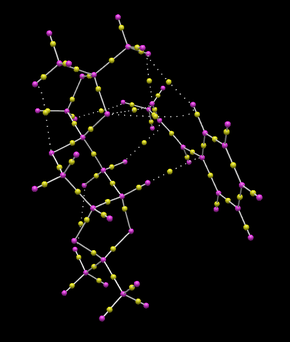 |
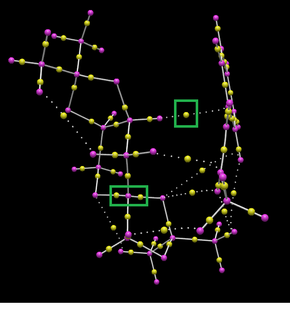 |
Bond critical points can be identified in the above diagram as yellow spheres. Interestingly some BCPs do not lie along bonds. Furthermore some do not lie exactly between nuclei as might be expected. In the right hand image BCPs have been highlighted with green boxes. The NCP located at the top right of the image does not lie in the place of a traditional covalent bond and is involved with the bond forming in the transition state of the reaction.
Two further BCPs (yellow spheres) have been highlighted in the green box towards the bottom left of the image. These do relate to covalent bonds but illustrate the assymetrical orientation of the BCP related to the bond atoms. This is caused by differences in atom electronegativity.
Suggested new candidates for the investigations
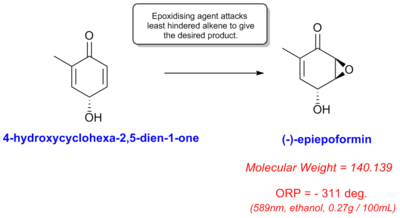
The compound (-)-epiepoformin was chosen as a new canditate for investigation. It's optical rotation has only been reported once in literature as -311 deg (Ethanol, 589nm, 0.27 g/100ml).[7]. A possible synthesis is shown for the compound by epoxidation of the least hindered alkene (i.e. the right hand alkene in the diagram on this page). Furthermore, it is likely the epoxide ring would be placed on the top of the molecule due to steric repulsions from the OH group pointing down in the starting material. This would ensure correct product stereochemistry.
Although the starting material shown is not currently available from Sigma Aldrich, it's synthesis has been achieved by a research group.[8]. Interestingly the starting material compound shows activity against renal and colon cancers.
References
- ↑ W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891. DOI:10.1021/ja00398a003
- ↑ C. S. Swindell, T. F. Isaacs, K. J. KanesTetrahedron Letters, 1985, 3, 289-292. DOI:10.1016/S0040-4039(01)80799-1
- ↑ 3.0 3.1 L. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. Rogers", J. Am. Chem. Soc., 1990, 112, 227–28.DOI:10.1021/ja00157a043
- ↑ R. Scott Rowland and Robin Taylor, J. Phys. Chem., 1996, 100 (18), 7384-7391. DOI:10.1021/jp953141+
- ↑ Z.X Wang, L. Shu, M. Frohn, Y. Tu, Y. Shi, Organic Syntheses, 2003, 80, 9. DOI:10.1002/0471264180.os080.02
- ↑ E. N. Jacobsen , W. Zhang , A. R. Muci ,J. R. Ecker , L. Deng J. Am. Chem. Soc., 1991, 113, 7063–7064; DOI:10.1021/ja00018a068
- ↑ David M. Pinkerton , Martin G. Banwell, Anthony C. Willis Org. Lett., 2009, 11 (19), 4290–4293; DOI:10.1021/ol9016657
- ↑ Wells G, Berry JM, Bradshaw TD, Burger AM, Seaton A, Wang B, Westwell AD, Stevens MF. J Med Chem., 2003, 13;46(4), 532–541; DOI:10.1021/ol9016657
