Rep:Mod:Brooklyn99
Computational chemistry is very much in its early stages, whereby its results are compared to experimental data, and any discrepancy between the two is always concluded to stem from an over-simplification on the part of the computational calculation. Despite this, when calculations do correlate well with experiment, insights into bonding energies, isomeric stability and other molecular features can be attained. For simple molecules, using small atoms, approximations do not drift far from reality, and accurate models can be analysed, to explain why certain conformations may dominate over others. It requires little effort to understand how much more economical it would be to reliably predict reaction outcomes without the need for 'wet' chemistry experiments. Analyses such as the ones conducted below do have their place in highlighting some of the limitations of computational chemistry, and the hurdles left to overcome before such chemistry can begin to dominate.
Part 1
Dicyclopentadiene
Using Avogadro 1.1, the most stable conformer was first calculated by comparision of the total energies of both the endo- and exo- conformers. An advantage of Avogadro, other than the fact it is freeware, is that it gives a breakdown of the energy contributions to the overall energy. This could be useful for cases where a particular property has a significant influence on the overall energy of the molecule. Avogadro 1.1 only uses classical mechanical optimisation techniques, and will not be as accurate as say the Gaussian package, which uses quantum mechanics.
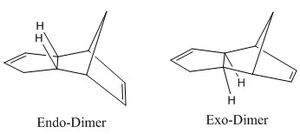
After preparation of a quantity of cyclopentadiene, dimerisation is a spontaneous reaction pathway, and can occur within a few hours if stored at room temperature. The specific reaction follows a [π4s + π2s] cycloaddition mechanism. It is worth noting that some computer simulators may find difficulty in assigning how many electrons come from each monomer, as the reacting molecules are identical, and be unable to continue. Woodward-Hoffmann rules invoke a 4n+2 electron movement and, assuming thermal activation, predict that the reaction proceeds via a transition state with Huckel topology. This can be evidenced in the models for the endo- and exo- dimers where the hydrogens along the transannular bond are cis- to one another (see figure 1 for a diagrammatic representation).
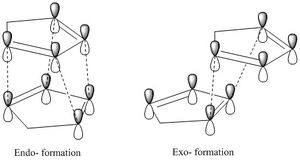
Sterically, the exo- dimer is less encoumbered, and many would predict this to be the thermodynamically favoured product. However, the endo-product is favoured under the usual laboratory conditions. An explanation for this has been found in the form of secondary orbital overlap (SOO), whereby favourable interactions from orbitals not immediately involved in the dimerisation, help stabilise the endo- transition state[1] (see figure 2). This rationale implies a kinetically stabilised endo- product.
Table 1 details the results for calculations run on Avogardo 1.1 with an MMFF94s force field and using a 'conjugate gradients' algorithm. From these results it is first useful to understand that the simulation uses interpolation by working within known data. Since anything in the way of transition states does not have such data associated with it, this software cannot calculate kinetic stability and does not provide a distinction between kinetics and thermodynamics. With this in mind, the total energy results are as expected from a thermodynamic stand-point. The exo- dimer appears thermodynamically more stable. In terms of the individual energy contributions, the angular bending term is the important factor in this calculation. Analysis of the two 'newly formed' bonds shows a C-C-C bond angle of 115.8o for the exo- product, and a 117.8o angle for endo- product. Considering an ideal tetrahedral bond angle of around 109o, the endo- product is furthest from ideality.
| Energy contributions | File:IMAGE of exo Dimer 1: Exo Dimer |
File:IMAGE of endo Dimer 2: Endo Dimer |
|---|---|---|
| Stretching energy (kcal mol-1) | 3.5429 | 3.46694 |
| Angular Bending energy (kcal mol-1) | 30.77292 | 33.19072 |
| Stretch Bending energy (kcal mol-1) | -2.04139 | -2.08182 |
| Torsional energy (kcal mol-1) | -2.73102 | -2.94913 |
| Van der Walls interactions (kcal mol-1) | 12.80138 | 12.35782 |
| Electrostatic energy (kcal mol-1) | 13.01372 | 14.18421 |
| Total energy (kcal mol-1) | 55.37344 | 58.17071 |
| Energy contributions | File:IMAGE of 3 3 |
File:IMAGE of 4 4 |
|---|---|---|
| Stretching energy (kcal mol-1) | 3.30447 | 2.82306 |
| Angular Bending energy (kcal mol-1) | 30.85205 | 24.68541 |
| Stretch Bending energy (kcal mol-1) | -1.92531 | -1.65717 |
| Torsional energy (kcal mol-1) | 0.08166 | -0.37832 |
| Van der Walls interactions (kcal mol-1) | 13.27369 | 10.63721 |
| Electrostatic energy (kcal mol-1) | 5.12107 | 5.14702 |
| Total energy (kcal mol-1) | 50.72286 | 41.25749 |
Partial hydrogenation of Dicyclopentadiene
Hydrogenation of a single olefin position is known, with full hydrogenation occurring over an extended period of time, or a larger excess of co-reagents[2]. To determine which of the two olefin positions provides the more favourable mechanism, Avogadro 1.1 was used to calculate the energies of the two possible products (see figure 3 for compounds 3 and 4), with the theory being that the lower energy product would be the single hydrogenation most likely to occur first. But once again, this approach will ignore any kinetic rates, and so reference to experimental results would help make sense of the data.
The calculated results in table 2 show that Dimer 4 is the lowest energy hydrolysed dicyclopentadiene. Comparing this to experimental preference found[3] indicates that dimer 4 is indeed the most favourable semi-hydrogenated product, owing to thermodynamic stability. Again, angle bending energy is the pivotal value in these results. Relief of bond strain from conversion of sp2 centres into sp3 centres is more pronounced in the case of dimer 4, it is more stabalised. Van Der Waals energy also has a notable influence. The hydrogens gained by molecule 4 are in close proximity to hydrogens on the opposite side of the ring, offering attractive Van Der Waals interactions that stabalise the molecule. Hydrogens added to molecule 3 do not have the right spacial geometry to have an equivalent stabalising effect. This results in a lower Van Der Waals energy for molecule 4.
Taxol Intermediates and Atropisomerism
Atropisomerism is a specific form of chemical isomerism in which two of the same compound differ in conformation due to high energy rotational barriers, restricting interconversion and allowing single atropisomers to be isolated. Using Avogadro 1.1, it is possible to manually adjust a molecule in real-time. In other words, the molecule may be stretched and skewed by the user, and the software will then work to position the molecule back into its most stable geometric position. This technique was used to manipulate Taxol intermediates, adjusting the cyclohexane ring to explore its atropisomers, as is explained below.
| Energy contributions | 9 (Chair) | 9 (Twist boat) | 10 (Chair) | 10 (Twist Boat) |
|---|---|---|---|---|
| Stretching energy (kcal mol-1) | 8.18060 | 8.35332 | 7.14326 | 7.25139 |
| Angular Bending energy (kcal mol-1) | 32.40116 | 32.01882 | 22.41039 | 23.21232 |
| Torsional energy (kcal mol-1) | -0.58061 | 2.23819 | -1.88221 | 1.16900 |
| Van der Walls interactions (kcal mol-1) | 35.39873 | 37.70051 | 32.11406 | 33.60176 |
| Electrostatic energy (kcal mol-1) | 0.43823 | 0.31935 | 0.83171 | 0.83810 |
| Total energy (kcal mol-1) | 76.46166 | 81.62709 | 61.03627 | 66.58689 |
The Oxy-Cope rearrangement is based on a [3,3]-Sigmatropic rearrangement and can be a very facile reaction[4]. Figure 3 shows the starting compound, and two possible atropisomers 9 and 10 resulting from an Oxy-Cope rearrangement. These two products were analysed using Avogadro 1.1, with a detailed summary included in table 3. In total, four atropisomers could be calculated as being a stable state for the molecule. Each of molecules 9 and 10 can be broken down into two further atropisomers, owing to the chair and twist boat conformations possible for the internal 6-membered ring.
| Energy contributions | Ketone 17 chair |
Ketone 18 | |
|---|---|---|---|
| Chair | Twist boat | ||
| Stretching energy (kcal mol-1) | 15.08489 | 14.39695 | |
| Angular Bending energy (kcal mol-1) | 30.79938 | 28.34193 | |
| Torsional energy (kcal mol-1) | 9.76748 | 13.77708 | |
| Van der Walls interactions (kcal mol-1) | 49.56976 | 50.54338 | |
| Electrostatic energy (kcal mol-1) | -6.11651 | -6.36955 | |
| Total energy (kcal mol-1) | 100.62772 | 102.20804 | |
An isolated cyclohexane ring experiences its lowest energy conformation in the chair state[5]. This is evidenced in the results found here, as in both cases (either ketone up or ketone down) the chair conformation sits at a lower total energy than the twist boat. The two larger energetic contributions that cause this were found to be torsional energy and Van Der Waals energy.

Di-thiols 17 and 18 (see figure 4), derived from Taxol, are more complex molecules, used here to test computational power and begin to expose limitations of the software. Table 4 summarises results using the same Avogadro 1.1 setup as before. Molecule 17 was resolved further using Gaussian, which also calculated NMR and vibrational data. In this way, the spectral data could be compared to literature in order to gauge how accurately the molecule had been modeled.
It was found that, whilst molecule 17 only existed in a single atropisomer, molecule 18 could form both a chair and twist boat conformation, as seen for Taxol intermediates 9 and 10. In continuing with the comparison to molecules 9 and 10, the 'ketone down' position, i.e. with carbonyl pointing away from the bridging alkyl groups, are always the thermodynamically more stable.
NMR Analysis
| 1H NMR | 13C NMR | |
|---|---|---|
| Spectrum |  |

|
Comparison of calculated1H NMR spectra with literature
| Computed δ(ppm) DOI:10042/28052 |
Integral | Reported Values[7] δ (ppm) |
|---|---|---|
| 5.15 | 1 | 4.84, (dd, J=7.2,4.7Hz, 1H) |
| 3.45 | 1 | 3.40-3.10 (m, 4H) |
| 3.29 | 2 | |
| 3.21 | 1 | |
| 3.02 | 2 | 2.99 (dd, J=6.8, 5.2 Hz, 1H) |
| 2.55 | 3 | 2.80-1.35 (Series of m, 14H) |
| 2.49 | 1 | |
| 2.28 | 4 | |
| 2.16 | 1 | |
| 2.08 | 1 | |
| 1.72 | 1 | |
| 1.67 | 1 | |
| 1.47 | 3 | |
| 1.35 | 1 | |
| 1.18 | 3 | 1.10 (s, 3H) |
| 1.10 | 3 | 1.00 (m, 1H) |
| Computed δ(ppm) DOI:10042/28052 |
Reported[8] δ (ppm) |
|---|---|
| 217.12 | 218.79 |
| 145.11 | 144.63 |
| 125.50 | 125.33 |
| 89.10 | 72.88 |
| 59.10 | 56.19 |
| 54.40 | 52.52 |
| 53.43 | 48.50 |
| 51.87 | 46.80 |
| 47.74 | 45.76 |
| 47.63 | 39.80 |
| 44.17 | 38.81 |
| 42.50 | 35.85 |
| 38.63 | 32.66 |
| 35.45 | 28.79 |
| 32.24 | 28.29 |
| 28.43 | 26.88 |
| 26.39 | 25.66 |
| 26.26 | 23.86 |
| 21.45 | 20.96 |
| 19.74 | 18.71 |
The calculated proton NMR spectrum shows some limitations. Gaussian has arbitrarily grouped protons of near-identical chemical shifts together as 'degenerate' sets. This is despite the protons being located several bonds apart, and in similar, but different environments. To work around this problem, the chemical shifts for protons belonging to each of the three methyl groups were averaged to form three new chemical shift values. These values were used in place of the 9 original methyl shifts, which will account for environmental averaging caused by rapid rotation about the methyl single bonds. This is more reminiscent of how a real molecule would behave.
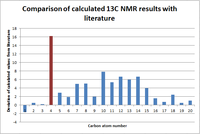
The calculated 13C spectrum correlates very well to literature values. The only notable exception is for the calculated 89.10 chemical shift due to the heavy atom effect (highlighted in figure 5). This refers to the carbon atom directly bonded to two sulphur atoms, which have more associated electrons and are more complex. For this reason, approximations used by Gaussian are adhered to more poorly than the lighter Hydrogen or even carbon atoms. It is possible to invoke pseudo-potentials, where the software models the inner electrons of a heavy atom as a single shell, and a valence set of electrons on top of this. However, it is inevitable that, as atom sizes increase, they become more complex and harder to model. This is an important computational limitation that limits computational chemists to only a certain number of compounds.
Part 2
The aim of this section is to provide an analysis of the crystal structure of the Jacobsen catalyst and the Shi catalyst, and to retrieve some information that will help explain the enantioselectivity of the catalysts. Crystal structure data was extracted from the Cambridge Crystal Database, and analysed using Mercury, an accompanying software package that allows the user to explore database structures and view crystal packing in 3D.
Shi Catalyst Precursur
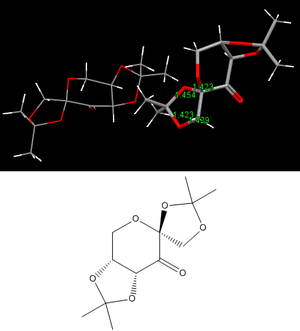
The fructose-derived ketone shown in figure 6 is the pre-catalyst first synthesised by Shi et. al.[9] used to epoxidise alkenes with a high degree of enantiomeric excess (ee), specifically for trans-substituted and trisubstituted alkenes[10]. Transition state analysis in a subsequent section provides some computational evidence for this. The crystal structure present in the same figure is one of two structures in the unit cell. Values are included for C-O bond lengths within one of the 5-membered rings, one of which appears to be larger than the expected (C-O) value of 1.42 Å[11]. The bond distance value of 1.454 Å is most likely as a result of a lone pair donation from the oxygen of the bond, into the adjacent σ*C-O orbital, weakening the C-O bond in question. The anomeric effect allows the equitorial position to be favoured over the axial, due to positive orbital overlap that stabalises the equitorial conformation. This effect is not observed for any of the other possible candidates for anomeric interactions, largely due to the electron widthdrawing influence of the carbonyl bond.
Jacobsen Catalyst Precursur
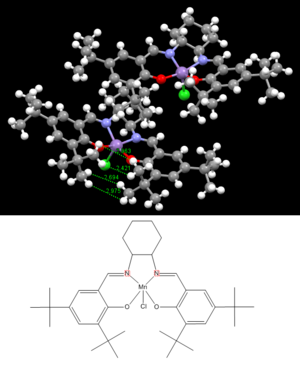
This metal-centred precatalyst favours the epoxidation of unfunctionalised alkyl- and aryl- compounds[12]. Again, the resulting catalyst appears to have high enantioselectivity. Favourable Van Der Waals (dispersion) forces are attractive forces that occur over longer-than bond-length distances. For two hydrogen atoms located approximately two Van Der Waals radii (2.4 ANGSTROMS) apart, an attractive force will be present, stabilising the molecule. Figure 7 contains the crystal structure for the Jacobsen catalyst, and highlighted are the H….H atom distances on the two tertiary butyl groups in close special proximity to one another. One of these distances was found to be 2.421 ANGSTROMS, an ideal distance for dispersion forces. Another interaction may exist where an interatomic distance of 2.694 ANGSTROMS is observed. The gap separating the two groups in question is quite possibly essential in restricting the active site areas on the catalyst, in other words, stopping attack from this angle.
Optical Rotation analysis
Certain analytical techniques may be modeled with computer software, with optical rotation analysis being one such method. Observation of the parity of the resulting value can show which enantiomer has been modeled, and allow the computational chemist to deduce which enantiomer they have made in the lab, by comparison to an experimentally measured valued. Making sense of the magnitude of these values, however, is less straight forward because of sensitivity issues with light conditions, temperature, pressure etc. Calculation of optical rotation values for β-methyl styrene oxide and 1,2-dihydronapthalene oxide. In order to carry out this calculation, each molecule was first drawn using ChemDraw 13.0, mechanically optimised with Avogadro 1.1 in the same way previously discussed and following this, a Gaussian optical rotation input file was generated. The Cambridge B3LYP density functional method was used, and the input file was constructed to ask for absorptions at 365nm and 589nm.
| Compound | Optical rotation (o) | |
|---|---|---|
| Calculated prediction | Literature Value | |
| (R)-styrene oxide | DOI:10042/28249 |
|
| (S)-styrene oxide | DOI:10042/28250 |
|
| ((R,S)-1,2-dihydronaphthalene oxide | DOI:10042/28248 |
|
| (S,R)-1,2-dihydronaphthalene oxide | DOI:10042/28251 |
|
From table 5, it is evident that calculations show strong correlation with literature results. Minor deviations are not important here, because the aim of this calculation was to assign R or S to each enantiomer, and prove that two seperate enantiomers exist. The S,R conformation for 1,2-Dihydronapthalene might at first seem anomalous, but upon inspection of the mathematics based around a 365o circle, 35.86o - 180o = -144.14o. Envisaging a sinosoidal curve with a phase length of 180o may help with this. The resulting value lies close to the literature value. Optical rotation values for 365nm are not currently present in literature, and no useful information has been attained in its regard.
Transition state analysis
Calculations have been computed for various transition states in an attempt to find the true geometry of reacting olefin with catalyst. The following section involves collecting free energy values for these transition states, determining which state is the lowest energy for each enantiomeric version of the olefin, and calculating enantiomeric excess values from the data. In this way, one can have an informed view on which catalyst has a preference for which enantiomeric configuration.
Shi Catalyst Transition States
From analysis of the transition state free energies calculated as a result of Shi epoxidation for β-Styrene Oxide, the lowest R,R transition state energy was found to be -1343.032443 a.u. The lowest S,S transition state energy was found to be -1343.024742 a.u. The overall lowest energy transition state, and therefore the preferred one, is the R,R variation. The difference in energy between these two diastereomers is a free energy (ΔG) of -20.218977 kJ/mol
Using a variation of the Gibbs Free Energy equation, ΔG = -RT ln K, a value for the equilibrium constant was calculated to be 3514.8.
Following this, the enantiomeric excess was calculated with the equation (K-1)/(K+1) x 100, where K is the rate constant calculated above. This yields a value of 99.94% enantiomeric excess. This means that 99.94% of the total yield is the R,R enantiomer.
This same calculation is also done with 1,2-Dihydronapthalene below:
Lowest energy R,S diastereomer = -1381.134059 a.u.
Lowest energy S,R diastereomer = -1381.136239 a.u.
ΔG= -5.723590 kJ/mol
K= 10.1
EE= 82.96% S,R enantiomer preferred.
Jacobsen Catalyst Transition States
The following results are from transition state analysis using β-Methyl Styrene:
Lowest energy R,S diastereomer = -3383.251060 a.u.
Lowest energy S,R diastereomer = -3383.259559 a.u.
ΔG= -22.3141262 kJ/mol
K= 8190.9
EE= 99.97% S,R enantiomer preferred.
The following results are from transition state analysis using 1,2-Dihydronapthalene:
Lowest energy R,S diastereomer = -3421.359499 a.u.
Lowest energy S,R diastereomer = -3421.369033 a.u.
ΔG= -25.0315189 kJ/mol
K= 24541.2
EE= 99.99% S,R enantiomer preferred.
It is clear that, energetically, certain transition states are preferred over others, leading to each catalyst preferring a substrate with a certain stereochemistry. Research through literature[17] tells a slightly different story. Enantiomeric excess percentages are much closer to 50% than the high values calculated above. As alluded to in the introduction, the computational approach will suffer from lack of detail, including no inclusion of possible side-reactions, not to mention many more potential transition states than the 4 main alignments calculated during this write-up. Often, large compounds may be simplified slightly, in order to make them computable, in order to give a less accurate, but informative result.
Further Study
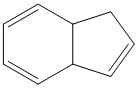
in order to analyse other epoxides, olefins with cheap precursurs would be ideal. Furthermore, known literature papers on specific epoxidation reactions may prove useful. The example given in figure 8 can be hydrogenated from the readily available indene. It may prove useful to compare this case to the previously discussed 1,2-Dihydronapthalene example.
References
- ↑ M. Fox, R. Cardona, N. Kiwiet, J. Org. Chem., 1987, 52 1469–1474. DOI:10.1021/jo00384a016
- ↑ Tetrahedron Letters, Vol. 37, No. 38, pp. 6925-6928, 1996 DOI:S0040-4039(96)01518-3
- ↑ Tetrahedron Letters, Vol. 37, No. 38, pp. 6925-6928, 1996 DOI:S0040-4039(96)01518-3
- ↑ L. Paquette, S. Elmore, Tetrahedron Letters, 1991, 32, 319-322. DOI:10.1016/S0040-4039(00)92617-0
- ↑ Nelson, Donna J.; Brammer, Christopher N. (2011). "Toward Consistent Terminology for Cyclohexane Conformers in Introductory Organic Chemistry". J. Chem. Ed. (American Chemical Society) 88 (3): 292–294. DOI:10.1021/ed100172k
- ↑ L. Paquete, N. Pegg, D. Toops, G. Maynard and R. Rogers, J. Am. Chem. Soc., 1990, 112, 277-283. DOI:10.1021/ja00157a043
- ↑ L. Paquete, N. Pegg, D. Toops, G. Maynard and R. Rogers, J. Am. Chem. Soc., 1990, 112, 277-283.DOI:10.1021/ja00157a043
- ↑ L. Paquete, N. Pegg, D. Toops, G. Maynard and R. Rogers, J. Am. Chem. Soc., 1990, 112, 277-283.DOI:10.1021/ja00157a043
- ↑ Zhi-Xian Wang, Yong Tu, Michael Frohn, Jian-Rong Zhang, and Yian Shi*, J. Am. Chem. Soc. 1997, 119, 11224-11235. DOI:10.1021/ja972272g
- ↑ Frohn, M.; Shi, Y. Synthesis 2000, 14, 1979-2000 DOI:10.1055/s-2000-8715 (Review)
- ↑ A. Bondi, J. Phys. Chem., 1964, 68, 441.
- ↑ Zhang, W.; Loebach, J. L.; Wilson, S. R.; Jacobsen, E. N. "Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes". J. Am. Chem. Soc., 1990, 112 (7): 2801–2803. DOI:10.1021/ja00163a052 .
- ↑ D. Forbes, S. Bettigeri, S. Patrawala, S. Pischek, M. Standen, Tetrahedron,, 2009, 65, 70-76. DOI:10.1016/j.tet.2008.10.019
- ↑ H. Lin, J Qiao, Y Liu, Z. Wu, J. Mol. Cat. B., 2010, 67, 236-241. DOI:10.1016/j.molcatb.2010.08.012
- ↑ D. Boyd, N. Sharma, R. Agarwal, N. Kerley, A. McMordie, A. Smith, H. Dalton, J. Blacker, G. Sheldrake, J. Chem. Soc. Chem. Comm., 1994, 14, 1693-1694. DOI:10.1039/C39940001693
- ↑ H. Sasaki, R. Irie, T. Hamada, K. Suzuki, T. Katsuki, Tetrahedron, 1994, 50, 11827-11838. DOI:10.1016/S0040-4020(01)89298-X
- ↑ J. Hanson, J. Chem. Educ., 2001, 78, 1266. DOI:10.1021/ed078p1266
