Rep:Mod:Bernaspage
NH3 Molecule:
Calculation method:
B3LYP, OPTF
Basis set:
6-31G(d,p
Final energy, E(RB3LYP) in atomic units (au):
-56.56 au
RMS gradient:
0.00000485
Point group of molecule:
C3V
N-H bond distance:
1.018 Angstroms
H-N-H bond angle:
105.74 Degrees
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES

NH molecule |
The optimisation file is liked to here

Modes expected using 3N-6 rule:
As there are 4 atoms, 6 modes are expected
Degenerate modes:
Modes 2 and 3 are degenerate with each other. 5 and 6 are also degenerate.
Bending vibrations:
1,2 and 3
Bond stretch vibrations:
4,5 and 6
Highly symmetrical mode:
4
Umbrella mode:
1
Bands expected to see in experimental spectrum of gaseous ammonia:
3
Charge distribution

A negative charge is expected for N as it is the most electronegative atom in the molecule. Furthermore, it has a lone pair of electrons which makes it more negative. As a result, the hydrogens have positive charges as the nitrogen pulls eletronegativity away from the hydrogens.
H2 Molecule
Calculation method:
B3LYP, OPTF
Basis set:
6-31G(d,p)
Final energy, E(RB3LYP) in atomic units (au):
-1.178 au
RMS gradient:
0.0972
Point group of molecule:
D∞h
H-H bond distance:
0.60 Angstroms
H-H bond angle:
180 Degrees
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES

H molecule |
The optimisation file is liked to here

Modes expected using 3N-6 rule:
As there are 2 atoms , 0 modes are expected
Degenerate modes:
N/A
Bending vibrations:
N/A
Bond stretch vibrations:
1
Highly symmetrical mode:
1
Umbrella mode:
0
Bands expected to see in experimental spectrum of gaseous hydrogen:
0
Charge distribution

This is expected as the electronegativity difference between the hydrogen atoms are equal therefore there is no polarisation.
N2 Molecule
Calculation method:
B3LYP, OPTF
Basis set:
6-31G(d,p)
Final energy, E(RB3LYP) in atomic units (au):
-109.52 au
RMS gradient:
0.0000006
Point group of molecule:
D∞h
N-H bond distance:
1.105 Angstroms
H-N-H bond angle:
180 Degrees
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES

N molecule |
The optimisation file is liked to here

Modes expected using 3N-6 rule:
As there are 2 atoms , 0 modes are expected
Degenerate modes:
N/A
Bending vibrations:
N/A
Bond stretch vibrations:
1
Highly symmetrical mode:
1
Umbrella mode:
0
Bands expected to see in experimental spectrum of gaseous nitrogen:
0
Charge distribution

This is expected as the difference between the electronegativities of nitrogen is 0. Therefore, the molecule is not polarised.
Energy of reaction N2 + 3H2 -> 2NH3
E(NH3)= -56.56
2*E(NH3)= -113.12
E(N2)= -109.52
E(H2)=-1.178
3*E(h2)= -3.53
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.07 x 2625.5 = -183.78kJ/mol
My Molecule: F2
Calculation method:
B3LYP, OPTF
Basis set:
6-31G(d,p)
Final energy, E(RB3LYP) in atomic units (au):
-199.43 au
RMS gradient:
0.232
Point group of molecule:
D∞h
N-H bond distance:
1.16 Angstroms
H-N-H bond angle:
180 Degrees
Item Value Threshold Converged? Maximum Force 0.000128 0.000450 YES RMS Force 0.000128 0.000300 YES Maximum Displacement 0.000156 0.001800 YES RMS Displacement 0.000221 0.001200 YES

F molecule |
The optimisation file is liked to here

Modes expected using 3N-6 rule:
As there are 2 atoms , 0 modes are expected
Degenerate modes:
N/A
Bending vibrations:
N/A
Bond stretch vibrations:
1
Highly symmetrical mode:
1
Umbrella mode:
0
Bands expected to see in experimental spectrum of gaseous ammonia:
0
Charge distribution

As the molecule has two of the same atom, which therefore have identical elecronegativities, it is expected to have no overall charge or polarisation as the electronegativity difference is 0.
MOs of F2:


The orbital on the left is the LUMO (lowest unoccupied molecular orbital) of F2. It is the 3σ* MO (molecular orbital) orbital created by the 2p atomic orbitals (AO) of the two flourine molecules. The orbital and antibonding and unoccupied by electrons.
The orbital on the right if the bonding orbital of the same MO. It is the 3σ MO, which is also made form the 2p orbitals of flourine. It does, however, contain electrons. The 3σ MO is much lower in energy than the 3σ* MO and the π* and π orbitals.
Both of these are the 3rd σ orbitals that are formed in F2.
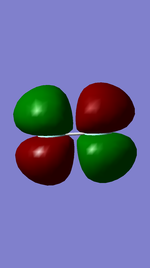

These are the π amd π* orbitals. The π* is the orbital shown on the left. It is the antibonding MO, which is also formed from 2p AO like the 3σ MO. The π* MO contains electrons, unlike the 3σ* orbital.
The π orbital, shown on the right, also contains electrons. It is also made from the 2p AO of flourine. The π MO is lower in energy than π*.
These are the first π orbitals formed in F2. There are two π and π* orbitals, in different axis (only one is shown).

This is the 2σ* MO of the F2 molecule. It is the second σ MO created in the F2 molecule. It is made by the 2s AO in the flourine atoms. The MO orbital shown is the antibonding orbital shown of the 2σ MO. It is lower in energy than the ones shown above.
Independent study: H2SiO
Calculation method:
B3LYP, OPTF
Basis set:
6-31G(d,p
Final energy, E(RB3LYP) in atomic units (au):
-365.90
RMS gradient:
0.000203
Point group of molecule:
D3h
Si - H bond distance:
1.48662 Angstrom
Si = O bond distance:
1.53123 Angstrom
H - Si = O bond angle:
124.110 Degrees
Item Value Threshold Converged? Maximum Force 0.000023 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.000023 0.001800 YES RMS Displacement 0.000017 0.001200 YES

HSiO molecule |
The optimisation file is liked to here

Modes expected using 3N-6 rule:
As there are 3 molecules, 3 vibrational modes are expected.
Degenerate modes:
There are no modes which are degenerate, some, however, are very close in energy.
Bending vibrations:
1 and 2
Bending and stretching vibrations:
3 and 4
Bond stretch vibrations:
5 and 6
Highly symmetrical mode:
1, 3 and 5
Umbrella mode:
N/A
Bands expected to see in experimental spectrum of gaseous H2SiO:
3
Charge distribution

A negative charge is expected for oxygen as it has lone pairs. Furthermore, it is much more electronegative than silicon, therefore it has an electron withdrawing effect. As a result it makes silicon more positive. Silicon then draws some electronegativity from the hydrogens, making them slightly negatively charged.
