Rep:Mod:BTW861
Introduction
In this exercise, the reaction pathways of several Diels Alder reactions and its transition states were studied. A Diels Alder reaction is a form of pericyclic reactions involving a [4+2] cycloaddition as well as the rearrangement of π bonds. The pathway that reactants undertake in a reaction can be modeled against its potential energy. Specifically,the Potential Energy Surface is mathematical function that plots the energy of a molecule against 2 degrees of freedom (e.g coordinates). Varying 1 coordinate would generate the energy profile diagram of a reaction.
A minimum on the Potential energy surface represent different 'stable' configurations the molecule can adopt. Motion in any direction would change the potential energy of the system. A local minimum represents a lowest energy point for a limited area on the PES. This is not the most stable conformation the molecule is able to adopt. The lowest point on the entire PES is known as the Global minimum and represents the most stable conformation of the molecule.The highest point on a reaction pathway is representative of a transition state and can be studied by locating the saddle point on a PES.A minimum point (representing a ground state) and transition state structures both have zero first derivative with respect to the PES.Taking the second derivative would allow us to differentiate between a minima and a transition structure.A mimima (ground state) has a positive second derivative while a transition state has a negative second derivative.By locating the transition states on the energy profile, the reaction barriers and reaction paths can also be calculated.
Transition states were modeled using Gaussian and geometry optimizations. Frequency calculation on Gaussian can be used to confirm the presence of a transition state.After the minimization of the transition state, only one negative frequency should appear and corresponds to the reaction.
Nf710 (talk) 09:24, 18 November 2016 (UTC) Nice intro, you could have spoke about the various methods in more detail.
Exercise 1: Reaction of Butadiene with Ethylene
Butadiene and Ethylene undergo a [4+2] cycloaddition reaction to yield cyclohexane as the product. The reaction involves the rearrangement of the pi system and formation of two new sigma bonds. Butadiene acts as the diene while Ethylene acts as the dienophile. This is an example of a 'normal electron demand' diels alder reaction in which th the HOMO of the diene reacts with the LUMO of the dienophile.3.This can be explained by consideration of the reactant's frontier molecular orbitals. The diagram below shows a Molecular orbital diagram for the cycloaddition reaction.
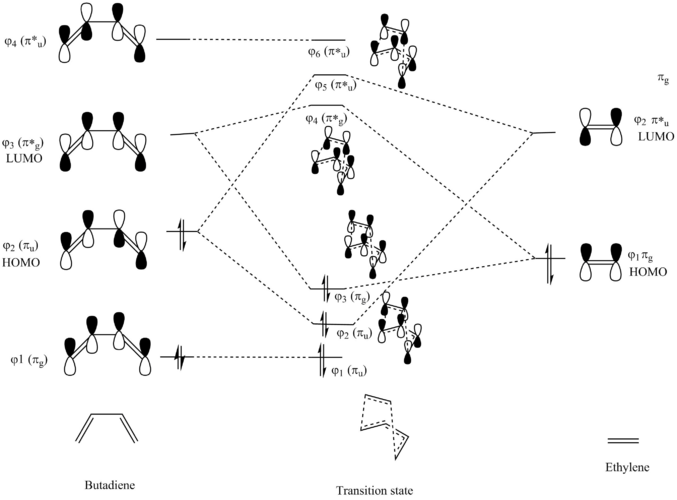
The 4 HOMO and LUMO orbitals of butadiene and ethylene combine to yield 4 new molecular orbitals in the transition state. From the diagram above there are two possible combinations between the fragment orbitals . In the first case, Ψ2 of the butadiene could be considered the HOMO and can interact with Ψ2) of ethylene which would act as the LUMO. Convesely, Ψ1 of ethylene could instead act as the HOMO while Ψ3 of butadiene acts as the LUMO. The first interaction is preferred as these frontier molecular orbitals are closer in energy. There is a larger interaction between these orbitals and results in a larger splitting when the new molecular orbitals are formed in the transition state.
When orbitals of similar phase combine, the electron density within that area increases. Overlap between orbitals of different phase lead to anti-bonding interactions and result in the formation of nodes. The greater the number of nodes, the higher the energy.The new molecular orbitals formed by the interactions between the HOMOS and LUMOS of the reactants are summarised in the table below:
| Ethylene MO | Butadiene MO | MO in Transition state | MO in Transition state | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
| ||||||||||||
| π*u | πu | πu | π*u | ||||||||||||
|
|
|
| ||||||||||||
|
|
|
| ||||||||||||
| πg | π*g | πg | π*g | ||||||||||||
|
|
|
|
It can be seen that fragment orbitals must have similar symmetry labels in order to undergo mixing to generate new molecular orbitals during a reaction. In this example, Ψ2 of butadiene and Ψ2 of ethene have πu symmetry labels and mix together, while Ψ3 of butadiene and Ψ1 of ethene have πg symmetry labels and likewise undergo orbital mixing.The extent of orbital overlap between two fragments(A and B) can be quantified by the overlap integral (SAB). It can be defined as:
The overlap between orbitals of similar symmetry result in a non zero overlap integral. Conversely, orbitals with different symmetry will not react and have an overlap integral equating to zero. The overlap integrals between different orbitals symmetries are summarised in the table below.
| Symmetry of Reacting Orbitals | Value of overlap integral | Reaction allowed/forbidden |
|---|---|---|
| Gerade-Gerade | Non zero | Allowed |
| Ungerade-Ungerade | Non zero | Allowed |
| Ungerade-Gerade | Zero | Forbidden |
Bond Length Measurements
| Type of Bond | Length (Å)3 | |
|---|---|---|
| 1. | sp3C- sp3C | 1.54 |
| 2. | sp3- sp2 | 1.50Å |
| 3. | sp2- sp2 | 1.33 |
| 4. | .Van Der Waals Radii | 1.70 |
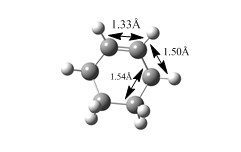
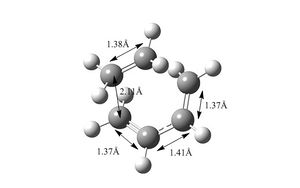
The carbon carbon bond lengths in the reactants and products were similar to typical carbon-carbon bond lengths. Bond lengths in butadiene C-C double bonds were approximately 1.33Å, C-C single bond was 1.47Å , slightly shorter than expected Carbon carbon bond length in ethylene is 1.32 Å which was expected. In the cyclohexene product, The single c-c bond lenghts measured out at 1.54 A while the C-C double bond measured out at 1.33 A. In the transition sate, many intermediate bond length between a single and double carbon bond were measured out. For example, the double bond in ethylene lengthened to 1.38 Å. In butadiene the single C-C shorened to 1.41Å, suggesting the formation some double bond character. The two new carbon bonds that would form between ethylene and butadiene measured out to be 2.11 A. Although this longer than a typical C-C single bond, it is shorter than 2 covalent radii of carbon ,suggesting the formation of bonding interactions at the reaction centre. The bond length measurements are shown in the diagrams below.

|

|
|
|
| Ethylene | Butadiene | Cyclohexene | Transition State |
Vibrational Analysis
|
| ||||||
| Negative Vibration (corresponding to transition state) | Lowest Positive Vibration |
The Diels Alder reaction between butadiene and ethylene is an example of a a synchronous concerted reaction and the two new bonds are formed simultaneously.This can be seen in the transition state where the the length of the new carbon bonds formed are the same. The vibrations of the transition state also show the formation of two new carbon-carbon bonds at the same time.1
Nf710 (talk) 09:33, 18 November 2016 (UTC) This section was done well. Not significant mistakes. Nice use of the overalap equation. and good use of Jmols
Exercise 2: Reaction of Benzoquinone with Cyclopentadiene
In this reaction, Benzoquinone with Cyclopentadiene undergo a diels alder reaction. This reaction produces two possible products, the exothermic and endothermic adduct. This is because there are two possible arrangements for Cyclopentadiene to adopt when in the s-cis position. The first conformation occurs when the diene and dienophile are aligned directly over each other.This arragement leads to the formation of the endo-adduct. The second confirmation occurs when the diene components "points away" from the dienophile. The reactants are staggered with respect to each other, resulting in the formation of the exo-adduct.
Nf710 (talk) 09:38, 18 November 2016 (UTC) Not exothermic, just exo.
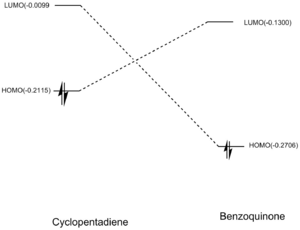
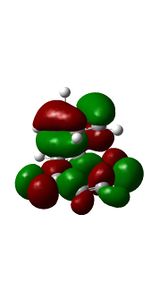
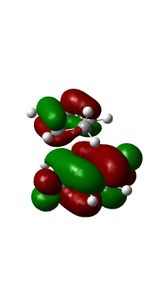
This is another example of a 'normal electron demand' diels alder reaction. Firstly, smaller energy gap between the HOMO cyclopendatiene and LUMO of Benzoquinone results in a more favourable overlap. Secondly, the HOMOS and LUMOS of the transition states have Πu and Π*usymmetry labels respectively. They have similar orbital symmetries correspond to Ψ2 and Ψ*5 of the transition state on the Diels-Alder MO diagram shown above. The contributing fragment orbitals can be traced back to the HOMO of the diene component and the LUMO of the dienophile component, thus indicaiting that the reaction follows the 'normal electron demand'
09:38, 18 November 2016 (UTC) Good understanding here!
| MO Diagram Orbitals | Exo TS HOMO | Endo Ts HOMO | MO Diagram Orbitals | Exo Ts LUMO | Endo TS LUMO |
|---|---|---|---|---|---|
 |
 |
 |
 |
 |
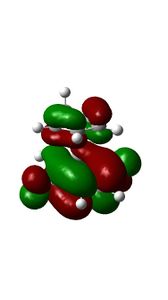 |
09:41, 18 November 2016 (UTC) Your HOMOs looks likek they have sigmna bonds in them. This is not your fault its becuase we are doing a DFT method.
All reactants, products and transition state were optimised using the PM6 method and refined with B3LYP/6-31G(d). The transition states for each reaction pathway were located and confirmed via a frequency calculation. A reaction coordinate was plotted and information regarding the reaction enthalpies and activation energies are tabulated in the table below.
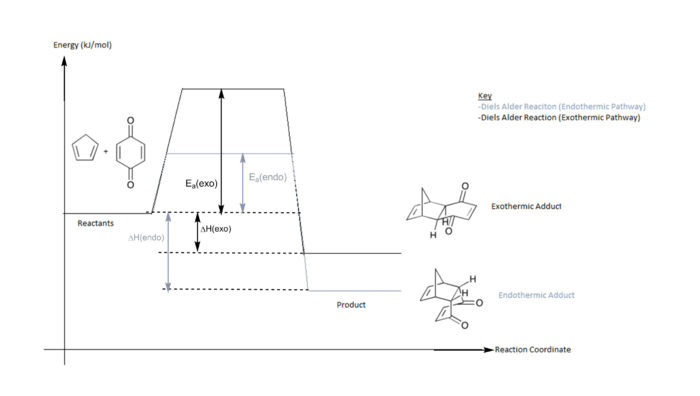
| Ea (kj/mol) | ΔH (kj/mol) | |
|---|---|---|
| Endothermic Transition state | 12.000 | -125.389 |
| Exothermic Transition state | 12.917 | -132.078 |
Despite having greater repulsive steric interactions,we can see that the endo-product is the kinetically and thermodynamically favoured. The endo adduct is favoured kinetically as it has a lower activation energy barrier.This is due to favorable secondary orbital overlap between Cyclopentadiene and Benzoquinone in the endothermic transition state. The 'eclipsed' conformation where the diene is directly above the dienophile. This allows for extra bonding interactions between the back lobes of the diene and the carbon atoms on Benzoquinine. This lowers the activation barrier in the endothermic transition state, allowing it to be obtained more quickly.The conformation of the exothermic transition state does not allow for extra overlap between Cp and Benzoquinone as seen in the image below.Despite being more sterically hindered, endo adduct is also the thermodynamically favoured product . This is because the stabilizing orbitals interactions present in the transition state can also be foundi in the product. The "attractive delocalization interaction" gained from secondary orbital interactions can "compensate for steric repulsion" causing it to be favoured thermodynamically.2
Nf710 (talk) 09:45, 18 November 2016 (UTC) This section was done very well, however you hav e got slightly wrong answers to your energy barriers, you have taken your reactants at infinate seperation, when it is better to minimise the first point on the IRC as there is an interaction energy. you have still got all the right conclusions so well done. very nicely formatted etc. and excellent arguement for the normal or inverse demand.
Exercise 3: Diels-Alder vs Cheletropic Reaction
In this reaction, o-xylyene and SO2 can under go either a Hetero-Diels Alder or cheletropic reaction. There are 3 different products that can be formed due to the different mechanisms (Endothermic Diels Alder, exothermic Diels Alder and cheletorpic reaction.) Both the Hetero-Diels Alder and cheleoptric reaction are examples of pericyclic reactions. The rearrangement σ and π bonds occurs in this cyclic array manner during the reaction. However the main difference with the cheletropic reaction is that both new bonds are being made on the same atom in one of the reactants. Eg in the reaction between xylylene and sulfur dioxide, the two new sigma bonds are being formed on the sulfur atom.
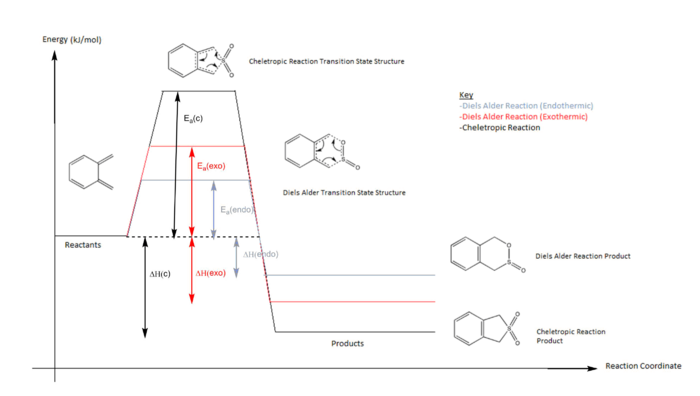
| ΔH (kj/mol) | Ea (kj/mol) | |
|---|---|---|
| Diels Alder Endothermic Adduct | -100.882 | 79.900 |
| Diels Alder Exothermic Adduct | -101.535969 | 83.884731 |
| Cheletropic Mechanism | -102.203 | 157.871 |
 |
 |
 |
| Cheletropic IRC | Endothermic Reaction IRC | Exothermic Reaction IRC |
o-Xylylene is highly unstable. From the IRC calculations of each reaction, we can see that the 6 membered ring gains aromatic character. This adds to the stabilization of the products that form at the end of the reaction
Extension
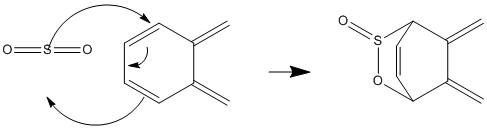
In the o-Xylylene reactant, there is a second s cis-butadiene fragment in the 6 memebered ring that can undergo a Diels-Alder reaction reaction. This reacts with SO2 to form a strained bridged hetro-cyclic compound. Although possible, Diels-Alder reaction at this site is thermodynamically and kinetically unfavorable.
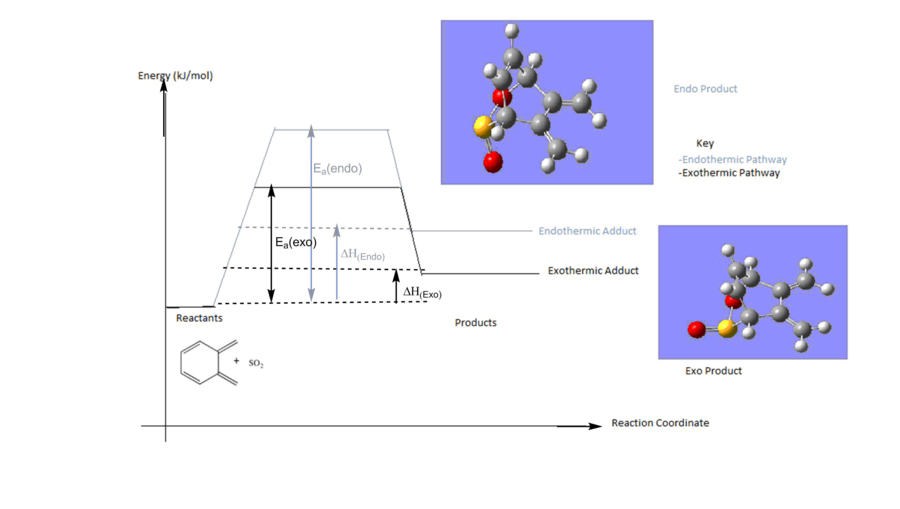
The reactants were calculated at the PM6 level and used to locate the endo and exo TS. A frequency calculation was used to confirm the presence of the transition state structure. The energies of the structures were tabulated and used to determine the reaction barriers and reaction energies at room temperature.
 |
 |
| Endothermic Reaction IRC | Exothermic Reaction IRC |
| ΔH (kj/mol) | Ea (kj/mol) | |
|---|---|---|
| Diels Alder Exothermic Adduct | 113.443 | 17.717 |
| Diels Alder Endothermic Adduct | 121.285 | 22.164 |
(The definition of endo and exo are retained - in the endo, the oxygen will overlap with the reacting diene Tam10 (talk) 13:43, 11 November 2016 (UTC))
From the IRC calculations, there are two possible trajectories for the SO2 to approach o-Xylylene . This results in the formation of the exothermic and endothermic adduct. These have different implications on the relative stability of products. Overall, the reaction between the 2nd diene component of o-Xylylene and SO2 is endothermic reaction with a very a large activation energy. The large kinetic barrier disfavours the reaction at this site. Moreover, the adducts formed from the reaction are thermodynamically less stable compared to the reactants. This could be due to the increase in steric clash in the product between the carbon atoms and the newly formed "sulfur-oxygen bridge". However, the exothermic product is slightly more stable in comparison to the endothermic adduct. This is due to the S=O bond which points away from the ring in the exo adduct, thus decreasing steric clashes with the 2nd diene component in Xylylene. The products formed in this reaction are not resonance stabilized,unlike the previous diels alder reaction.
(Stabilisation might instead come from overlap of p-orbitals between the oxygen and the newly formed alkene Tam10 (talk) 13:43, 11 November 2016 (UTC)(
Conclusion
In this exercise, Diels Alder reaction pathways were studied and its transition state characterized. The approach in which reactants adopt during the reaction can have significant effects on the transition state structure as well as the product. Steric hindrance can either increase or decrease depending on how reactants approach each other. Moreover depending on the overlap of the reactants in the transition state, secondary orbital interactions may be possible. This can lower the energy barrier of the transition state and affect the predicted outcome of a reaction. E.g in exercise 2, the exo-adduct would be predicted to the the major product as it is sterically less hindered, but favourable secondary orbital interactions in the endo transition state favour the endo adduct causing it to be the major product of the reaction.
Bibliography
References
1.D. LR, Organic Chem Curr Res, 2013, 02.
2.C. Tormena, V. Lacerda Jr. and K. Oliveira, J. Braz. Chem. Soc., 2010, 21, 112-118.
3.S. Warren and J. Clayden, Organic chemistry, Oxford University Press, Oxford, 2001.