Rep:Mod:BT3418
Ammonia
Basic physical properties
Molecular formula: NH3
Bond length: N-H: 1.01798Å
Bond angle: H-N-H: 105.74115°
Optimisation
Calculation method: RB3LYP
Basis Set: 6-31G(d,p)
Charge: 0
Spin: Singlet
E(RB3LYP): -56.55776873 a.u.
RMS Gradient Norm: 0.00000485 a.u.
Dipole Moment: 1.8466 Debye
Point Group: C3V
Proof of converging:
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
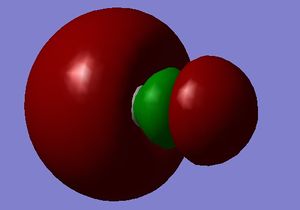
3D model
Ammonia NH3 |
Vibrational characters
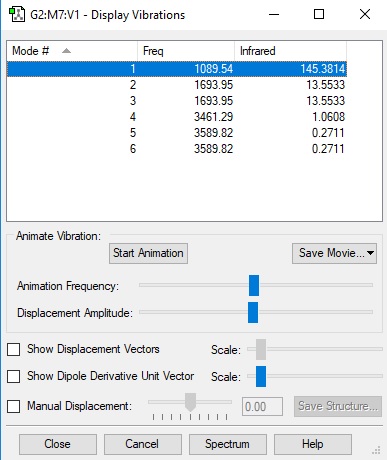 Display vibration table of ammonia.
Display vibration table of ammonia.
| Wavenumber(cm-1) | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity(Arbitrary units) | 145 | 14 | 14 | 1 | 0 | 0 |
| Image | 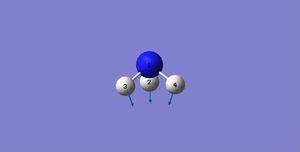
|

|
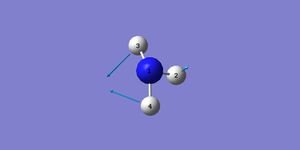
|
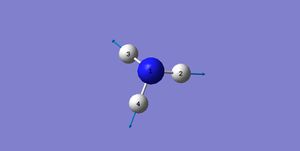
|

|
|
Questions and answers:
Q: How many modes do you expect from the 3N-6 rule?
A: Six vibrational modes.
Q: Which modes are degenerate (ie have the same energy)?
A: Mode 2&3, mode5&6.
Q: Which modes are "bending" vibrations and which are "bond stretch" vibrations?
A: Modes 1&2&3 are bending vibrations, while modes 4&5&6 are stretching vibrations.
Q: Which mode is highly symmetric?
A: Mode 4.
Q: One mode is known as the "umbrella" mode, which one is this?
A: Mode 1.
Q: How many bands would you expect to see in an experimental spectrum of gaseous ammonia?
A: Two bands according to the IR spectrum.
Atomic charge
Nitrogen atom: -1.125
Hydrogen atom: 0.375
 Atomic charge diagram for ammonia.
Atomic charge diagram for ammonia.
Nitrogen
Basic physical properties
Molecular formula: N2
Bond length: N-N triple bond: 1.10551Å
Bond angle: /
Optimisation
Calculation method: RB3LYP
Basis Set: 6-31G(d,p)
Charge: 0
Spin: Singlet
E(RB3LYP): -109.52412868 a.u.
RMS Gradient Norm: 0.00001600 a.u.
Dipole Moment: 0.0000 Debye
Point Group: D*H
Proof of converging:
Item Value Threshold Converged? Maximum Force 0.000028 0.000450 YES RMS Force 0.000028 0.000300 YES Maximum Displacement 0.000009 0.001800 YES RMS Displacement 0.000012 0.001200 YES
3D model
Nitrogen N2 |
Vibrational character
 Display vibration table of nitrogen.
Display vibration table of nitrogen.
| Wavenumber(cm-1) | 2457 |
| Symmetry | SGG |
| Intensity(Arbitrary units) | 0 |
| Image | 
|
Atomic charge
Nitrogen atom: 0.000
 Atomic charge diagram for nitrogen.
Atomic charge diagram for nitrogen.
Hydrogen
Basic physical properties
Molecular formula: H2
Bond length: H-H: 0.74289Å
Bond angle: /
Optimisation
Calculation method: RB3LYP
Basis Set: 6-31G(d,p)
Charge: 0
Spin: Singlet
E(RB3LYP): -1.17853935 a.u.
RMS Gradient Norm: 0.00003809 a.u.
Dipole Moment: 0.0000 Debye
Point Group: D*H
Proof of converging:
Item Value Threshold Converged? Maximum Force 0.000066 0.000450 YES RMS Force 0.000066 0.000300 YES Maximum Displacement 0.000087 0.001800 YES RMS Displacement 0.000123 0.001200 YES
3D model
Hydrogen H2 |
Vibrational character
 Display vibration table of hydrogen.
Display vibration table of hydrogen.
| Wavenumber(cm-1) | 4464 |
| Symmetry | SGG |
| Intensity(Arbitrary units) | 0 |
| Image | 
|
Atomic charge
Hydrogen atom: 0.000
 Atomic charge diagram for hydrogen.
Atomic charge diagram for hydrogen.
Structure and reactivity
Nitrogen
The bond length of the N-N triple bond in nitrogen molecule is 1.10551Å, while the bond length in [(Bp(CF3)2)RuH(iPr2NH)]2(N2), ABATIG, is 1.138Å. [1]
- ↑ Rodriguez, V.; Atheaux, I.; Donnadieu, B.; Sabo-Etienne, S. and Chaudret, B. 2000, 'Ruthenium Dihydridobis(pyrazolyl)borate Complexes Adopting a κ3 N,N,H, κ2 N,H, or κ2 N,N Bonding Mode', Organometallics, 19(15), pp 2916–2926. DOI: 10.1021/om000199o.
The N-N triple bond was stretched by approximately 0.033Å, which is caused by two dative covalent bonds to neutralise the repulsion and stereo hindrance between the two giant ligands. Due to the strength of the N-N triple bond, with a bond energy of 942 kJ/mol, the increase of the bond length is not very significant.
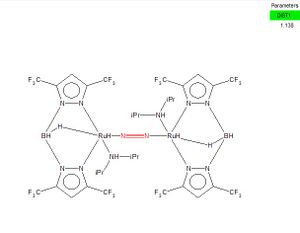 [(Bp(CF3)2)RuH(iPr2NH)]2(N2), ABATIG
[(Bp(CF3)2)RuH(iPr2NH)]2(N2), ABATIG
Hydrogen
The bond length of the H-H bond in hydrogen molecule is 0.74289Å, while the bond length in RuCl(SiMe3-nCln)(η2-H2)(PCy3)2, HIHVIE, is 1.050Å. [1]
- ↑ Lachaize, S.; Caballero, A.; Vendier, L. and Sabo-Etienne, S. 2007, 'Activation of Chlorosilanes at Ruthenium: A Route to Silyl σ-Dihydrogen Complexes', Organometallics, 26(15), pp 3713–3721. DOI: 10.1021/om700295g.
The H-H bond was stretched by approximately 0.307Å, which is caused by the decrease of the electronic orbital overlap of two hydrogen atoms. As a result, the attraction between two hydrogen atoms is decreased and the distance between the hydrogen atoms is lengthened to neutralize the electrostatic repulsion. The bond strength of the H-H bond is not as strong as N-N triple bond, with a bond energy of only 432 kJ/mol. As a result, the increase of the H-H bond, by approximately 41%, is significant.
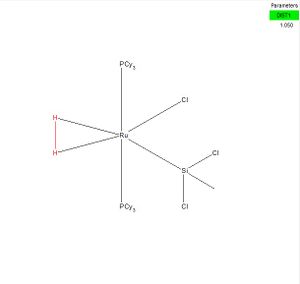 RuCl(SiMe3-nCln)(η2-H2)(PCy3)2, HIHVIE
RuCl(SiMe3-nCln)(η2-H2)(PCy3)2, HIHVIE
Discussion on the Haber-Bosch process
Reaction equation:
N2 + 3H2 -> 2NH3
E(NH3)= -56.55776873 a.u. = −148492.4 kJ/mol (to 1 decimal place)
2*E(NH3)= -296984.8 kJ/mol
E(N2)= -109.52412868 a.u. = -287555.6 kJ/mol (to 1 decimal place)
E(H2)= -1.17853935 a.u. = -3094.3 kJ/mol (to 1 decimal place)
3*E(H2)= -9282.9 kJ/mol
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -146.3 kJ/mol
The enthalpy change of the reaction is negative, so the reaction is exothermic. As a result, the internal energy of the gaseous reactants is higher than the internal energy of the product, which is ammonia gas. So, the product is more stable than the gaseous reactant.
Carbon monoxide
Basic physical properties
Molecular formula: CO
Bond length: C-O triple bond: 1.13794Å
Bond angle: /
Optimisation
Calculation method: RB3LYP
Basis Set: 6-31G(d,p)
Charge: 0
Spin: Singlet
E(RB3LYP): -113.30945314 a.u.
RMS Gradient Norm: 0.00000433 a.u.
Dipole Moment: 0.0000 Debye
Point Group: C*V
Proof of converging:
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000008 0.000300 YES Maximum Displacement 0.000003 0.001800 YES RMS Displacement 0.000004 0.001200 YES
3D model
Carbon monoxide CO |
Vibrational character
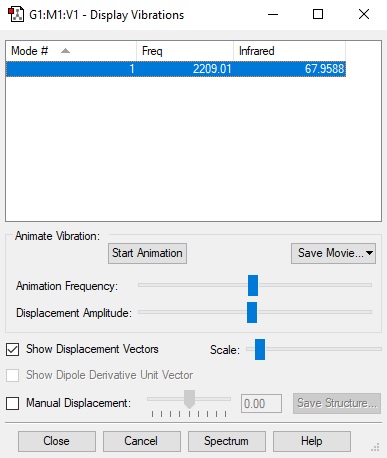 Display vibration table of carbon monoxide.
Display vibration table of carbon monoxide.
| Wavenumber(cm-1) | 2209 |
| Symmetry | SG |
| Intensity(Arbitrary units) | 68 |
| Image | 
|
Atomic charge
Carbon atom: 0.506
Oxygen atom: -0.506
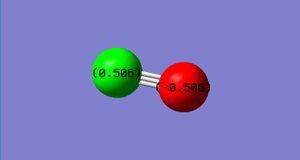 Atomic charge diagram for carbon monoxide.
Atomic charge diagram for carbon monoxide.
Molecular orbital of carbon monoxide
M.O. 1
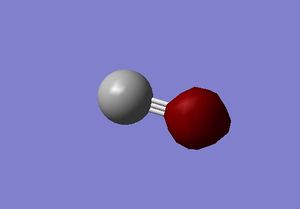 First molecular orbital of carbon monoxide.
First molecular orbital of carbon monoxide.
The first molecular orbital of carbon monoxide is the combination of two 1s core atomic orbitals of the carbon and oxygen atom. It is an occupied bonding orbital. The orbital is deep in energy, with an energy of -19.26 a.u. Inserting electros in this orbital can stabilise the bonding and form a σ bond between the two atoms.
M.O. 2
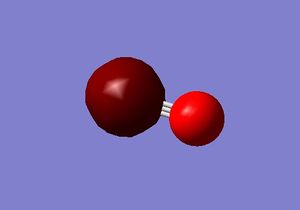 Second molecular orbital of carbon monoxide.
Second molecular orbital of carbon monoxide.
The second molecular orbital of carbon monoxide is the combination of two 1s core orbitals of the carbon and oxygen atom. It is an occupied anti-bonding orbital. the orbital is deep in energy, with an energy of -10.30 a.u. Inserting electros in this orbital will decrease the stability of the bonding between the two atoms and form a σ* bond.
M.O. 3
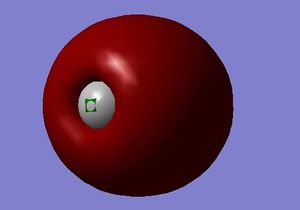 Third molecular orbital of carbon monoxide.
Third molecular orbital of carbon monoxide.
The third molecular orbital of carbon monoxide is the combination of two 2s atomic orbitals of the carbon and oxygen atom. It is an occupied bonding orbital. The orbital is deep in energy, with an energy of -1.16 a.u. Inserting electros in this orbital can stabilise the bonding and form a σ bond between the two atoms. The shape of the orbital shows an oxygen-dominant character in the molecule.
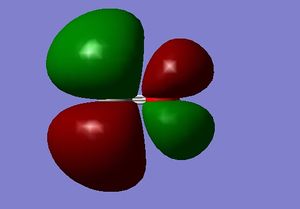
M.O. 7
 Seventh molecular orbital of carbon monoxide.
Seventh molecular orbital of carbon monoxide.
The seventh molecular orbital of carbon monoxide is the combination of two 2s atomic orbitals of the carbon and oxygen atom. It is an occupied orbital but a mixture of bonding and antibonding, with an energy of -0.37 a.u. Inserting electros in this orbital can stabilise the bonding between the two atoms. The shape of the orbital shows a 2s-2s mixing between the carbon and oxygen atom and an oxygen-dominant character as well. This orbital is also the highest occupied molecular orbital (HOMO) of the molecule, which has the highest energy among other occupied molecular orbitals.
M.O. 8
 Eighth molecular orbital of carbon monoxide.
Eighth molecular orbital of carbon monoxide.
The eighth molecular orbital of carbon monoxide is the combination of two 2p atomic orbitals of the carbon and oxygen atom but in a g conformation. It is an unoccupied orbital but a mixture of bonding and antibonding with an orbital, with an energy of -0.02 a.u. Inserting electros in this orbital will decrease the stability of the bonding between the two atoms and form a π* bond. In reverse, no electron is in fact inserted into this orbital, which can increase the bonding strength between the carbon and oxygen atoms. This orbital is also the lowest unoccupied molecular orbital (LUMO) of the molecule, which has the lowest energy among other unoccupied molecular orbitals.
Further investigation: The combustion of carbon monoxide.
Reaction equation:
2CO + O2 -> 2CO2
E(CO2)= -188.58093945 a.u. = -495119.3 kJ/mol (to 1 decimal place)
2*E(CO2)= -990238.6 kJ/mol
E(O2)= -150.25742434 a.u. = -394500.9 kJ/mol (to 1 decimal place)
E(CO)= -113.30945314 a.u. = -297494.0 kJ/mol (to 1 decimal place)
2*E(CO)= -594988.0 kJ/mol
ΔE=2*E(CO2)-[E(O2)+2*E(CO)]= -749.7 kJ/mol
The enthalpy change of the reaction is negative, so the reaction is exothermic. As a result, the internal energy of the gaseous reactants is higher than the internal energy of the product, which is carbon dioxide gas. So, the combustion product is more stable than the gaseous reactant.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 0/1
Have you completed the calculation and given a link to the file?
NO -You missed to include a link to the .log file of your finished calculation. This reduces the achievable mark for this section by 1.
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
NO - You missed to include a link to the .log file of your finished calculation. This reduces the achievable mark for this section by 1.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES - you should have rounded after the final calculation of the reactions energy. Due to that your result is wrong by 0.2 kJ/mol.
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 2/5
Have you completed the calculation and included all relevant information?
NO - You missed to include a link to the .log file of your finished calculation. This reduces the achievable mark for this section by 1.
Have you added information about MOs and charges on atoms?
YES
You could have explained the calculated vibrational modes and atomic charges. The first two MOs are both based on one 1s orbital each. MO1 on the 1s of O and MO2 on the 1s of C. Both are non-bonding as there is no overlap with another AO. You missed to explain the relative order of the MOs regarding energy (number of nodes...) MO7 shows two nodes. It cannot be build from two s orbitals and has definitely contributions from p AOs. MO8 can be identified as anti-bonding without any doubt.
Independence 0.5/1
If you have finished everything else and have spare time in the lab you could:
Check one of your results against the literature, or
Do an extra calculation on another small molecule, or
YES - To calculate the combustion of CO was a good idea! But you missed to include a link to the optimised structure of O2. But you are rewarded half a mark for searching crystal structures for both N2 and H2.
Do some deeper analysis on your results so far
