Rep:Mod:BMWWiki
Benjamin Wild Wiki, Module 1
The Basic Techniques of Molecular Mechanics and Semi-Empirical Molecular Orbital Methods for Structural and Spectroscopic Evaluations
Cyclopentadiene

1,3-Cyclopentadiene is key in a number of reactions (including Diels-Alder reactions), being useful in both synthetic chemistry and understanding the mechanistic aspects of cycloaddition due to its tendency to dimerise into dicyclopentadiene (right)[1]. An example of a reaction using cyclopentadiene is one undertaken in our second year labs at Imperial which involved the synthesis of ferrocene. The yield obtained for ferrocene was dependent on the time after cracking of dicyclopentadiene due to dimerisation of cyclopentadiene at room temperature (for this reason, after cracking the cyclopentadiene was chilled to reduce the rate of dimerisation).
Dimerisation to dicyclopentadiene occurs via a [4πs+2πs] cycloaddition (Diels-Alder), forming a possible two isomers (exo or endo, right). This reaction is kinetically controlled, favouring the endo product. There are several reasons hypothesised for this kinetic endo selectivity, which ultimately prescribe the reason to lie with orbital symmetry. When forming the endo product, the intermediate has better overlap between the HOMO and LUMO orbitals of each ring, and better plane-to-plane orientation for accumulation of double bonds[2].
Hydrogenation of Cyclopentadiene Dimers
Using molecular modelling methods in ChemBio3D, one is able to study each dimer and each hydrogenated version of the endo product to compare the relative stabilities of each. Below are diagrams illustrating each dimer, with an interactive option for each linked below respectively.
| Exo Cyclopentadiene Dimer 1 | Endo Cyclopentadiene Dimer 2 | Endo Cyclopentadiene Dimer 3 Pentene Ring Hydrogenation |
Endo Cyclopentadiene Dimer 4 Hexene Ring Hydrogenation |
|---|---|---|---|
Below are the calculated values for each dimer using Allinger MM2 molecular mechanics as the chosen method for optimising their geometries (all values reported are to 3 s.f.).
| Dimer | Minimised Energy |
Stretch kcal/mol |
Bend kcal/mol |
Stretch-Bend kcal/mol |
Torsion kcal/mol |
Non-1,4 VdW kcal/mol |
VdW kcal/mol |
Dipole/ Dipole kcal/mol |
|---|---|---|---|---|---|---|---|---|
| 1 | 31.9 | 1.29 | 20.6 | -0.838 | 7.66 | -1.42 | 4.23 | 0.378 |
| 2 | 34.0 | 1.25 | 20.8 | -0.836 | 9.51 | -1.54 | 4.32 | 0.448 |
| 3 | 31.2 | 1.10 | 14.5 | -0.549 | 12.5 | -1.07 | 4.51 | 0.141 |
| 4 | 35.9 | 1.23 | 18.9 | -0.761 | 12.1 | -1.50 | 5.73 | 0.163 |
From the above obtained results, one can see that thermodynamically, the exo isomer is the most stable (by approximately 2.1 kcal/mol). Difference in energy between isomers can mainly be attributed to 1,4 relationships between atoms, resulting in strain. By analysis of dihedral angles, it can be seen that dimer 1, in antiperiplanar conformation, suffers less strain in comparison to dimer 2, in synclinal conformation (179° and 46° respectively). This leads to the exo product being lower in energy, and therefore more thermodynamically stable in comparison to the endo product. Despite this, the kinetic endo product still predominates due to the reasons stated above.
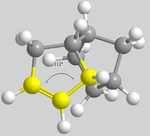
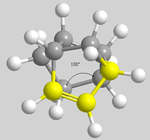
In comparing the two hydrogenated products (3 and 4), it can be seen that 3 (with the left hand ring hydrogenated) is lower in energy than 4 (with the right hand ring hydrogenated), meaning that 3 is the thermodynamic product. The difference in energies cannot be rationalised through 1,4 relationships as before due to the fact that the have the same dihedral angle of 51°. By rotating the molecules, the terminal dihedral angles can be compared regarding the angles around the double bonds (below).
| 3 | 4 |
|---|---|
|
|
From the above described angles, it can be seen that dimer 3 has a wider angle, and therefore less bond strain, than dimer 4 which. This corresponds to the results obtained, with dimer 3 being the thermodynamic product.
Taxol Intermediates

In the synthesis of taxol, a powerful anti-cancer therapy drug (specifically ovarian) originally isolated from the Pacific yew tree[3], there are two important intermediate isomers, with the carbonyl pointing up or down (illustrated on the right). Intermediates 9 & 10 are formed from an oxy-Cope rearrangement (thought of as an irreversible rearrangement)[4], and are examples of atropisomerism, with the compound appearing to isomerise between the two alone.
These isomers exist by trapping the configuration sterically (because of steric hindrance) through restricted rotation about a single bond. This was done theoretically using ChemBio3D by moving the bonds into positions which trapped these isomers, and refining model using MM2 minimisation. Initially, each isomer was trapped in the twist-boat conformation by the calculation; but by refining the atom positioning and bond lengths, it was possible to trap the cyclohexane ring of each isomer in the chair conformation, which is a more stable conformation (each of which are illustrated below).
Exo Taxol Intermediate (9A) |
Exo Taxol Intermediate (9B) |
Endo Taxol Intermediate (10A) |
Endo Taxol Intermediate (10B) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
Below is a table of results obtained using Allinger MM2 molecular modelling minimisation, and MMFF94 minimisation, for the above isomers. Alternative methods cannot be directly compared due to the different parameters set out in their computation, however methods of the same type can be.
| Intermediate | Minimised Energy |
Minimised Energy |
Stretch kcal/mol |
Bend kcal/mol |
Stretch-Bend kcal/mol |
Torsion kcal/mol |
Non-1,4 VdW kcal/mol |
VdW kcal/mol |
Dipole/ Dipole kcal/mol |
|---|---|---|---|---|---|---|---|---|---|
| 9A | 53.3 | 76.3 | 2.95 | 17.2 | -0.501 | 21.3 | -1.42 | 14.5 | -1.73 |
| 9B | 47.8 | 70.5 | 2.78 | 16.5 | -0.430 | 18.3 | -1.55 | 13.1 | -1.72 |
| 10A | 46.4 | 66.3 | 2.71 | 11.7 | 0.323 | 21.9 | -2.10 | 13.9 | 46.4 |
| 10B | 42.7 | 60.6 | 2.62 | 11.3 | 0.343 | 19.7 | -2.16 | 12.9 | -2.00 |

Both the MM2 and MMFF94 from the above results confirm that the chair conformation is the most stable for each isomer, and both these methods also confirm isomer 10 as the thermodynamically most stable of the isomers. The thermodynamic stability of isomer 10 can be rationalised by σ-π* conjugation between the carbon's σ orbital and the carbonyl's π* orbital. With the carboyl pointing down, there is a greater possible overlap between the two orbitals (illustrated to the right) when compared with the carbonyl pointing up[5].
These taxol intermediates are highly unreactive, and fall under a class of olefins known as "hyperstable" olefins. They fall under this unbrella term due to the level of their strain in comparison to the parent (saturated) hydrocarbon, due to the positioning of the double bond which offers a more cage like structure and decreases reactivity[6].
Modelling Using Semi-Empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
![[π+2π] Cycloaddition of Dichlorocarene to compound 12](/images/thumb/c/cf/12_cycloadd.png/300px-12_cycloadd.png)
The [π+2π] cycloaddition of dichlorocarbene to compound 12 (right) can be analysed theoretically using computational techniques that model the energy of 12's orbitals, and their forms displayed graphically. In doing this, one can illustrate orbital control of reactivity to determine the regioselectivity of dichlorocarbene's addition by modelling molecular orbitals using MOPAC/PM6 calculations in ChemBio3D.
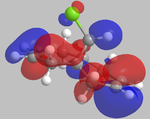
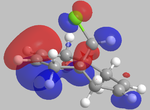
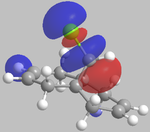
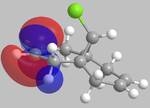
It can be seen from the below images that in the HOMO orbital, the olefinic bond syn to the chlorine is most susceptible to electrophilic attack. This is because it has a high electron density in comparison to the anti olefinic bond, therefore acting as a good electron donating group to incoming electrophiles. From the LUMO orbitals, it is clear that the olefinic bond anti to the chlorine has the largest vacant π* orbital compared to the syn olefinic bond. This makes the anti olefinic bond more susceptible to nucleophilic attack as it would be a better electron acceptor. From this, it is clear that the cycloaddition of dichlorocarbene to compound 12 would occur at the syn olefinic bond due to the strong electrophilic nature of dichlorocarbene.
Further justification of the syn selectivity can be seen by looking at the LUMO+1, and HOMO-1 orbitals. In the LUMO+1 orbital, the σ* of the C-Cl bond can clearly be seen to orientate towards the anti olefinic bond. By comparing the HOMO-1 orbital, it can be seen that the anti olefinic bond has a high π electron density directed towards where the σ* of the LUMO+1 lies, allowing for orbital mixing which stabilises the C=C π bonding orbital, and destabilises the C-Cl σ* bonding orbital. This gives further justification as to why the syn olefinic bond is the one more likely to be attacked (anti is more stabilised, therefore less open to electrophilic attack).
| HOMO-1 | HOMO | LUMO | LUMO+1 | LUMO+2 |
|---|---|---|---|---|
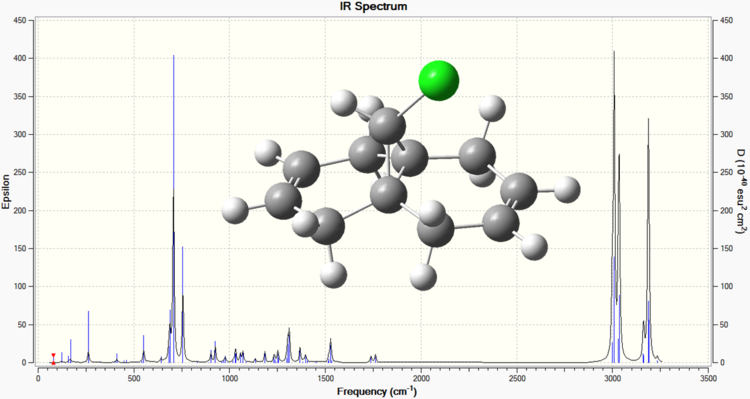
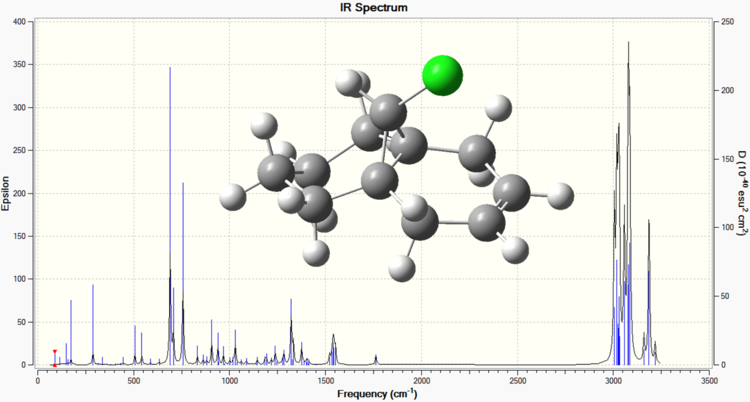
Below are the calculated IR spectra of compound 12, and compound 12 hydrogenated at the anti position respectively. These were obtained by subjecting the two compounds to a B3LYP/6-31G Gaussian geometry optimisation and frequency calculation (performed using Imperial's SCAN facility).
| Dialkene Compound 12 IR Spectrum | Monoalkene Compound 12 IR Spectrum |
|---|---|
From the above spectra, the following values of interest can be extracted[7].
| Bond | Frequency in Dialkene (wavenumber, cm-1) | Frequency in Monoalkene (wavenumber, cm-1) |
|---|---|---|
| C-Cl | 755.90 | 756.90 |
| C=Cexo | 1739.00 | Not present |
| C=Cendo | 1761.55 | 1762.85 |
These results confirm the differences between the two molecules, with the first spectrum having peaks for each olefinic bond, whereas the second spectrum only having one olefinic bond peak. The difference in the two C-Cl peaks can be attributed to the mixing of the LUMO+1 C-Cl σ* and the HOMO-1 anti C=C π which destabilises the C-Cl due to greater electron density being present in the σ*. A less stabilised bond would lower the characteristic frequency, and so the dialkene has a lower frequency for C-Cl than the monoalkene, because the monoalkene has the anti olefinic bond removed, and so the C-Cl bond is less destabilised.
Monosaccharide Chemistry: Glycosidation
Glycosidation is the process which involves replacing a specified group with an incoming nucleophile. There is possibilities in sugars to produce different anomers, depending on the orientation of the sugar ring's substituent groups. The main factor in producing a specific anomer is the neighbouring group effect. In the below, the neighbouring group effect is mainly directed by the ester group in the beta position from the ring oxygen. The beta anomer (C) allows attack from the top face of the oxonium cation, whereas the alpha anomer (D) allows attack from the bottom face of the oxonium cation. Below is the assigned glycosidation to analyse, in which the R groups used for latter calculations are methyl groups.
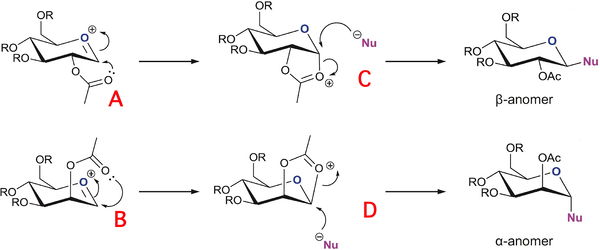
The 1,2-trans relationship of products formed from the neighbouring group effect is well grounded, with high stereochemical selection. However, it is reported that it is still common to obtain products whose purity is still not 100%[8]. This is demonstrated by the compounds below A & A', B & B', C & C', D & D'. The interactive illustrations have been calculated using Mopac/PM6 calculations.
| Monosaccharide A | Monosaccharide A' | Monosaccharide B | Monosaccharide B' | Monosaccharide C | Monosaccharide C' | Monosachharide D | Monosachharide D' | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
Below is the technical data obtained for the above molecules using MM2 and Mopac/PM6 calculations in ChemBio3D.
| Monosaccharide | Minimised Energy |
Minimised Energy |
Stretch kcal/mol |
Bend kcal/mol |
Stretch-Bend kcal/mol |
Torsion kcal/mol |
Non-1,4 VdW kcal/mol |
VdW kcal/mol |
Charge/ Dipole kcal/mol |
Dipole/ Dipole kcal/mol |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 22.9 | -91.7 | 2.84 | 11.1 | 1.05 | 2.01 | 1.10 | 18.6 | -19.9 | 6.04 |
| A' | 34.9 | -77.4 | 2.59 | 11.7 | 1.01 | 1.48 | -2.66 | 18.2 | -2.27 | 4.84 |
| B | 21.8 | -88.7 | 2.85 | 11.4 | 1.02 | 2.01 | 0.894 | 18.4 | -21.0 | 6.23 |
| B' | 33.9 | -77.5 | 2.67 | 9.96 | 0.890 | 4.01 | -1.60 | 19.5 | -5.83 | 4.26 |
| C | 27.8 | -91.6 | 1.94 | 13.1 | 0.678 | 8.52 | -2.98 | 18.0 | -9.00 | -2.47 |
| C' | 44.1 | -67.0 | 2.74 | 21.16 | 0.884 | 7.27 | -2.26 | 18.0 | -2.58 | -1.11 |
| D | 23.9 | -90.5 | 1.84 | 19.5 | 0.764 | 9.48 | -2.21 | 18.0 | -23.7 | 0.289 |
| D' | 46.4 | -66.8 | 2.79 | 18.8 | 0.939 | 9.31 | -2.36 | 18.9 | -1.18 | -0.822 |
Neighbouring group effects aren't factored into the Allinger MM2 calculations, and therefore the MM2 results are less useful in geometry optimisation. Mopac/PM6 calculations do however factor in such effects, and so make the modelling process more representative of the molecule. In comparing the Mopac/PM6 values in the above table, it is apparent that A&B, A'&B', C&D, C'&D' all have very similar values. This shows that there is very little energy difference between each respective couple, and that there is a high likelihood of each occurring. There is a definite difference between A and A', B and B' of approximately 20 kcal/mol for each. A' and B' are both lower than A and B respectively due to the fact that there is less steric strain, and so the complexes are more stable (lower in energy).
Structure Based Mini-Project Using DFT-Based Molecular Orbital Methods
The Total Synthesis of (-)Cubebol
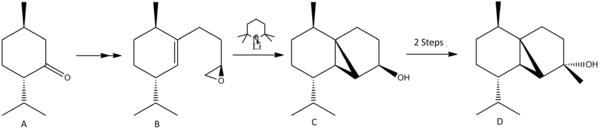
From its derivation of cubeb grown in the Indonesian archipelago, which has had medicinal and culinary uses, cubebol has been patented by Firmenich as a cooling/ refreshing agent in flavours (stronger than menthol, can provide a similar effect as ice-cream without the need to chill the product). Right is the overall synthesis of the molecule[9].
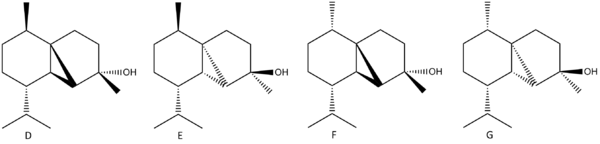
In the synthesis of (-)Cubebol, there are many possibilities for producing an isomer of a different geometry, though the route leading to (-)Cubebol will be the predominant one. One such way the geometry could be altered is if the epoxide of compound B was facing down rather than up. This would lead to the ring formation occurring from the under side rather that the top, with the stereochemistry being inverted in comparison (illustrated by D & E).
If (+)menthol is the precursor to making (+)menthone, then (-)-10-Epicubebol will form (alkyl group points down rather than up). This is useful to look at to compare with (-)Cubebol in terms of the differing characteristics between the two. The synthesis of (-)-10-Epicubebol follows a similar pathway as the route taken to synthesise (-)Cubebol, and so the same epoxide alteration in the synthesis steps as described above will used to compare compounds F & G.
NMR Analysis
Below is a table containing the literature 13C NMR data, compared with compounds being looked into (D-G). The literature data directly applies to both D and F, whilst E and G are compared with D and F respectively. The stated error in the calculation of the NMR spectra (MM2-->Mopac/PM6-->Gaussian) is said to be approximately 5ppm.
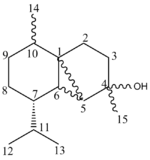
| Carbon no | δliterature[10] | D: δcalculated[11] | E: δcalculated[12] | F: δcalculated[13] | G: δcalculated[14] |
|---|---|---|---|---|---|
| 1 | 80.3 | 76.8 | 76.0 | 76.3 | 76.8 |
| 2 | 44.1 | 38.0 | 40.0 | 38.2 | 40.3 |
| 3 | 39.0 | 36.2 | 40.6 | 42.5 | 33.7 |
| 4 | 36.3 | 49.0 | 49.3 | 49.3 | 48.9 |
| 5 | 33.6 | 29.8 | 33.6 | 30.2 | 33.3 |
| 6 | 33.4 | 39.6 | 39.0 | 37.8 | 40.8 |
| 7 | 31.7 | 23.7 | 33.4 | 27.3 | 30.0 |
| 8 | 30.8 | 30.1 | 32.4 | 31.7 | 30.4 |
| 9 | 29.5 | 38.8 | 33.2 | 33.4 | 39.2 |
| 10 | 27.9 | 29.1 | 29.0 | 29.4 | 29.1 |
| 11 | 26.5 | 19.1 | 26.4 | 22.8 | 21.5 |
| 12 | 22.5 | 34.1 | 36.0 | 36.4 | 34.2 |
| 13 | 20.1 | 20.0 | 21.6 | 21.8 | 18.9 |
| 14 | 19.6 | 22.0 | 20.0 | 22.3 | 20.0 |
| 15 | 18.7 | 20.3 | 20.4 | 20.3 | 20.6 |
From this data, one can see that although not exactly fitting the literature values in each case, there is a general concordance between literature and the calculated NMR peaks. There is no literature data for molecules E and G, however there are significant differences in the 13C NMR spectra between D & E, and F & G. The main notable difference in NMR data between D and E is at C-7. This is likely to be due to hindrance between C-7 and the right hand ring. In D, C-7 and the ring are closer spacially due the arrangement of the R groups on the left hand carbon ring. In E, because of the bulky R group being in the same orientation (down) as the right hand ring, they not interact as strongly, pushing up the ppm. Comparing the literature data to each of these calculated sets of data, I am inclined to say that the NMR data is in fact more concordant with compound E than D. A similar effect is seen with F and G, with the major difference being with C-3. This is again due to orientation, with the lower ppm being with the right hand ring pointing up (and both R groups pointing down).
IR Analysis
Below is a table of data comparing literature and calculated IR data (calculated using a Gaussian calculation). The calculated values require a correction factor of 8% (systematically too high).
| Bond | D: νliterature[9] | D: νcalculated | D: νcorrected | E: νcalculated | E: νcorrected | F: νcalculated | F: νcorrected | G: νcalculated | G: νcorrected |
|---|---|---|---|---|---|---|---|---|---|
| Broad -OH stretch | 2961-2872 | 2990-3183 | 2751-2928 | 2979-3173 | 2740-2919 | 2991-3179 | 2751-2924 | 2978-3171 | 2739-2917 |
| -C-H bend | 1455, 1369, 1315 |
1537, 1519, 1436 |
1414, 1397, 1321 |
1537, 1508, 1436 |
1414, 1387, 1321 |
1539, 1517, 1435 |
1415, 1395, 1320 |
1537, 1516, 1437 |
1414, 1394, 1322 |
| =C-H bend | 750 | Main peak 869, range 806-989 |
Main peak 799, range 741-909 |
Main peak 878, range 769-976 |
Main peak 807, range 707-897 |
Main peak 874, range 767-991 |
Main peak 804, range 705-911 |
Main peak 871, range 808-984 |
Main peak 801, range 743-905 |
Below is are the IR spectra associated with each stereoisomer.
| D | E | F | G |
|---|---|---|---|
 |
 |
 |
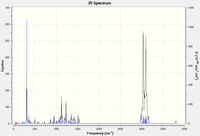 |
The calculated data seems to fit nicely with the data supplied in the literature, although not perfect. The data confirms the functional groups seen in D-F. Peaks assigned as =C-H bend correspond to the carbons at C-1 and C-5 due to their locked positions.
Optical Rotation
This would have been a good technique to look into further, and performed the calculations for due to the chiral nature of these molecules. Due to time constraints and problems with generating an iput file for SCAN that worked, I was unable to use this as an analytical technique.
References
- ↑ A. Kumar & S. Pawar, AlCl3-catalysed dimerization of 1,3-cyclopentadiene in the chloroaluminate room temperature ionic liquid, Journal of Molecular Catalysis A: Chemical, 208 (2004), 33-37
- ↑ http://www.ch.ic.ac.uk/motm/porphyrins/introDA.html
- ↑ K. Nicolaou et al, Total synthesis of taxol, Letters to Nature, The Scripps Research Institute, Vol 367 1994, 630-634
- ↑ S. Elmore & L. Paquette, The first thermally-induced retro-oxy-cope rearrangement, Tetrahedron Letters, Evans Chemical Laboratories, Vol 32 1991, No 3, 319-322
- ↑ http://www.ch.ic.ac.uk/local/organic/conf/
- ↑ W. Maier & P. Schleyer, Evaluation and prediction of the stability of bridgehead olefins, J. Am. Chem. Soc., 103 (1981), 1891-1900
- ↑ http://www2.ups.edu/faculty/hanson/Spectroscopy/IR/IRfrequencies.html?pagewanted=all
- ↑ D. Whitfield & T. Nukada, DFT studies of the role of C-2-O-2 bond rotation in neighbouring-group glycosylation reactions, Elsevier, Carbohydrate research, 342 (2007), 1291-1304
- ↑ 9.0 9.1 D. Hodgson et al, Stereocontrolled synthesis of (-)Cubebol and (-)-10-Epicubebol involving intramolecular cyclopropanation of α-lithiated epoxides, J. Org. Chem., 75 (2010), 2157-2168
- ↑ B. Wu et al, Antifeedants against Locusta migratoria from the Japanese Cedar, Cryptomeria japonica II, Biosci. Biotechnol. Biochem., 72 (2008), 611-614
- ↑ http://hdl.handle.net/10042/to-9544
- ↑ http://hdl.handle.net/10042/to-9545
- ↑ http://hdl.handle.net/10042/to-9546
- ↑ http://hdl.handle.net/10042/to-9547