Rep:Mod:BMG1417
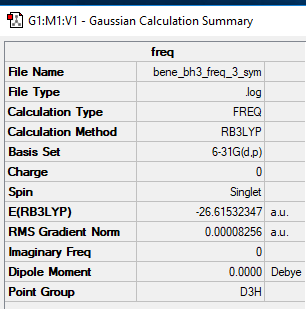
BH3
Computational Level and Basis Set: B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000165 0.000450 YES RMS Force 0.000083 0.000300 YES Maximum Displacement 0.000650 0.001800 YES RMS Displacement 0.000325 0.001200 YES
Frequency analysis log file File:BENE BH3 FREQ 33 SYM.log
Low frequencies --- -0.2407 -0.1105 -0.0054 44.9603 46.0936 46.0942 Low frequencies --- 1163.6314 1213.6102 1213.6129
BH3 molecule |
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163.63 | 92 | A2" | yes | out-of-plane bend |
| 1213.61 | 14 | E' | yes | bend |
| 1213.61 | 14 | E' | yes | bend |
| 2580.06 | 0 | A1' | no | symmetric stretch |
| 2713.01 | 126 | E' | yes | asymmetric stretch |
| 2713.02 | 126 | E' | yes | asymmetric stretch |
The vibrations shown in the spectrum are only three, though the ones observed in Guassview Frequency table are six. Out of these six, the symmetrical stretch (2580.06 cm-1) is IR inactive because it does not involve a change in dipole, so disobeys the selection rules. Of the remaining five, there two sets of degenerate vibrations (1213 cm-1 and 2713 cm-1) so that out of these four we only see one signal.
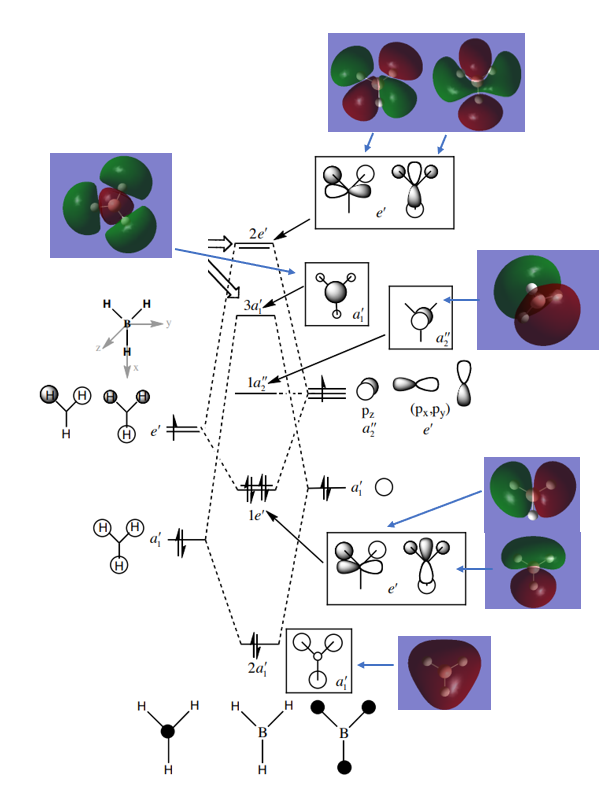
Molecular Orbital Diagram for BH3
Questions:
Are there any significant differences between the real and LCAO MOs?
The contributions from each FOs to the MOs is more accurate in the computer. Most importantly, the computed MOs show the overall electron density of the overlap rather than simply placing the orbitals drawings on top of each other. Going up in energy, however, this makes them harder to interpret.
What does this say about the accuracy and usefulness of qualitative MO theory?
MO theory is very accurate in showing the actual electron density of the FOs in-phase and out-of-phase overlaps.
Association Energies: Ammonia-Borane
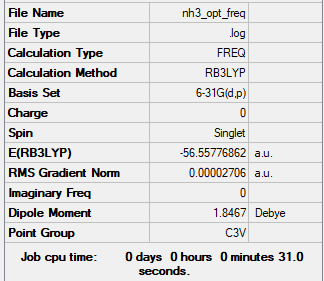
NH3
Computational Level and Basis Set: B3LYP/6-31G level
Item Value Threshold Converged?
Maximum Force 0.000050 0.000450 YES
RMS Force 0.000027 0.000300 YES
Maximum Displacement 0.000156 0.001800 YES
RMS Displacement 0.000083 0.001200 YES
Frequency analysis log file File:NH3 OPT FREQ2.LOG
Low frequencies --- -29.6300 -29.6158 -12.8157 -0.0034 0.0088 0.0562 Low frequencies --- 1089.6456 1694.1055 1694.1059
NH3 molecule |
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163.63 | 92 | A2" | yes | out-of-plane bend |
| 1213.61 | 14 | E' | yes | bend |
| 1213.61 | 14 | E' | yes | bend |
| 2580.06 | 0 | A1' | no | symmetric stretch |
| 2713.01 | 126 | E' | yes | asymmetric stretch |
| 2713.02 | 126 | E' | yes | asymmetric stretch |
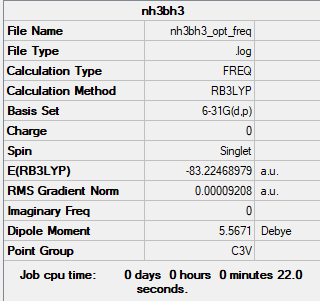
NH3BH3
Computational Level and Basis Set: B3LYP/6-31G level
Item Value Threshold Converged? Maximum Force 0.000209 0.000450 YES RMS Force 0.000092 0.000300 YES Maximum Displacement 0.001227 0.001800 YES RMS Displacement 0.000663 0.001200 YES
Frequency analysis log file File:NH3BH3 OPT FREQ.LOG
Low frequencies --- -0.0590 -0.0428 -0.0066 23.0658 23.0711 41.6772 Low frequencies --- 265.1032 632.3076 639.8596
NH3 molecule |
Vibrational spectrum for BH3
| wavenumber (cm-1 | Intensity (arbitrary units) | symmetry | IR active? | type |
| 1163.63 | 92 | A2" | yes | out-of-plane bend |
| 1213.61 | 14 | E' | yes | bend |
| 1213.61 | 14 | E' | yes | bend |
| 2580.06 | 0 | A1' | no | symmetric stretch |
| 2713.01 | 126 | E' | yes | asymmetric stretch |
| 2713.02 | 126 | E' | yes | asymmetric stretch |
Association Energy
E(NH3) = -56.55777 au
E(BH3) = -26.61532 au
E(NH3BH3) = -83.22469 au
Association Energy = ΔE =E(NH3BH3)-[E(NH3)+E(BH3)] = -83.22469 - (-56.55777 -26.61532) = -0.05160 au = -135.45 kJ/mol
Questions
Based on your energy calculation is the B-N dative bond weak, medium or strong? What comparison have you made to come to this conclusion?
Th B-N bond is 1.66804 units whereas the C-C bond in ethane is 1.50025 units.