Rep:Mod:BER1234
Introduction to Molecular Modelling
NH3
| Component | Result |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 631-G(d.p) |
| Final energy in a.u. | -56.5576873 a.u. |
| RMS gradient | 0.00000485 a.u. |
| Point group | C3v |
| Optimised NH bond length | 1.01798 Å |
| Optimised H-N-H bond angle | 105.741° |
Item table:
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
NH3 molecule |
File:BDAVIES NH3 OPTIMISATION.LOG
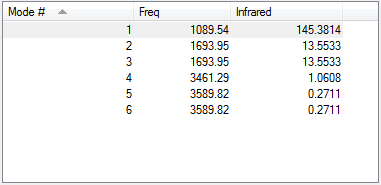
Vibration Analysis
| Number of modes expected from the 3N-6 rule | 6 |
| Degenerate modes | #5 and #6, and #2 and #3 |
| Bending vibrations | #1, #2 and #3 |
| Bond stretch vibrations | #4, #5 and #6 |
| Highly symmetric mode | #4 |
| Umbrella mode | #1 |
| Bands expected in an experimental spectrum of gaseous ammonia | 2 |
Charge Analysis
Charge on N atom = -1.125
Charge on H atom = 0.035
I would expect a positive charge on the H atoms and a negative charge on the N atom due to nitrogen being more electronegative than hydrogen and attracting more of the electron density in the N-H bond.
N2
| Component | Result |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 631-G(d.p) |
| Final energy in a.u. | -109.52412868 a.u. |
| RMS gradient | 0.00000060 a.u. |
| Point group | D(inf)H |
| Optimised N-N bond length | 1.10550 Å |
Item table:
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
File:BDAVIES N2 OPTIMISATION.LOG
N2 molecule |
There is only one vibrational frequency: 2457.33 cm-1
H2
| Component | Result |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 631-G(d.p) |
| Final energy in a.u. | -1.17853936 a.u. |
| RMS gradient | 0.00000017 a.u. |
| Point group | D(inf)H |
| Optimised H-H bond length | 0.74279 Å |
Item table:
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
File:BDAVIES H2 OPTIMISATION.LOG
H2 molecule |
There is also only one vibrational frequency for H2: 4465.68 cm-1
Energies for the Haber-Bosch Process
E(NH3) = -56.5576873 a.u.
2*E(NH3) = -113.1153746 a.u.
E(N2) = -109.52412868 a.u.
E(H2) = -1.17853936 a.u.
3*E(H2) = -3.53561808 a.u.
ΔE = 2*E(NH3)-[E(N2)+3*E(H2)] = -0.05562784 a.u. = -146.0508939 kJ/mol
Literature value for ΔE ~-50 kJ/mol(Prezhdo, 2016).
The ammonia product is more stable than the gaseous reactants.
Citation
Prezhdo, V. V. (2016). Haber Process Made Efficient by Hydroxylated Graphene: Ab Initio. The Journal of Physical Chemistry Letters.
Chosen molecule: F2
| Component | Result |
|---|---|
| Calculation method | RB3LYP |
| Basis set | 631-G(d.p) |
| Final energy in a.u. | -199.49825218 a.u. |
| RMS gradient | 0.00007365 a.u. |
| Point group | D(inf)H |
| Optimised F-F bond length | 1.40281 Å |
Item table:
Item Value Threshold Converged? Maximum Force 0.000128 0.000450 YES RMS Force 0.000128 0.000300 YES Maximum Displacement 0.000156 0.001800 YES RMS Displacement 0.000221 0.001200 YES
File:BDAVIES F2 OPTIMISATION.LOG
F2 molecule |
There is one vibrational frequency: 1065.09 cm-1. It is a stretch vibration. I would expect to see one band in the gaseous spectrum of flourine, corresponding to its one vibrational frequency.
There is 0 charge on each F atom, because it is a single-element molecule: therefore the electrons in the covalent bond are distributed equally.
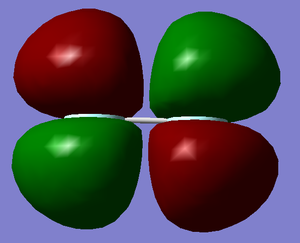
P orbital A
Energy = -0.58753 a.u.
This is the occupied δ bonding orbital. It is formed with two Pz atomic orbitals, one from each flourine atom.
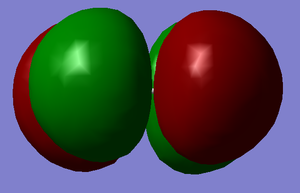
P orbitals B and C
Energy = -0.52332 a.u. These are the occupied bonding pi orbitals. They are formed with one px and one py atomic orbital from each flourine atom.

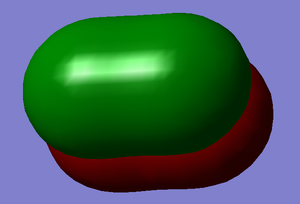
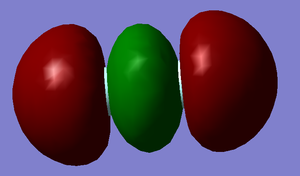
P orbitals D and E
Energy = -0.39190 a.u. These are the occupied anti-bonding pi orbitals. They are also formed with one px and one py atomic orbital from each flourine atom. These are the HOMOs.
The LUMO is the unoccupied δ* orbital, not shown here.