Rep:Mod:BE411
Molecular Modelling can be used to predict many properties of a system including its lowest energy conformation, its NMR spectra and it's optical rotation at specific wavelengths. This experiment will show this through a range of experiments looking at the dimerisation of cyclopentadiene, spectroscopy simulation of a taxol synthesis intermediate and analysis of the Shi and Jacobsen catalysised epoxidation reactions, focusing on both the epoxides themselves and their transition states. All energy optimisations were run using the Avogadro program and are reported in kcal/mol, these values cannot be quantitively compared to any other thermodynamic values and so are only discussed in terms of a qualitative comparison within this report.
The Hydrogenation of the Cyclopentadiene Dimer
The aim of this experiment is to look at the molecular mechanics of the cyclodimerisation of cyclopentadiene and the hydrogenation of dicyclopentadiene to discover if they're thermodynamically or kinetically controlled. This is achieved by running optimization calculations on the possible products of the reactions to identify product energies and comparing this data to that in the literature, giving detailed information on the specific contributions of the different energies within the molecule. The energies considered within the total energy are the bond stretching energy, the angle bending energy, the stretch bending energy, the torsional energy, the out-of-plane bending energy, the van de Waals energy and electrostatic energy.
- Bond Stretching Energy: the potential energy allowing a bond to be compressed or stretched
- Angle Bending Energy: the potential energy to allow for bond angles to change from their equilibrium position
- Stretch Bending Energy:the potential energy required to relieve strain from the change in bond length to counteract a decrease in bond angle
- Torsional Energy: energy to rotate about bonds intramolecularly in order to change conformation
- Out-of-Plane Bending Energy: potential energy to cause a change in the angle between a bond and the plane of the molecule
- Van der Waals Energy: the through-space interaction between two non-bonded atoms which can be either attractive or repulsive
- Electrostatic Energy: this energy considers all dipoles, and their interactions, within a molecule
Dimerisation of Cyclopentadiene
Dicyclopentadiene is formed by a Diels-Alder Reaction between two cyclopentadiene molecules [1]. Diels-Alder reactions are a [4+2] cycloaddition between a diene and dienophile to form 6-membered systems with regio and stereochemical control. It is an example of a bipericyclic mechanism as it occurs through both a [3,3] sigamtropic shift as well as π2s + π4s and a π4s + π2s cycloaddition[2]. Due to its concerted nature the reactions are stereospecific giving the syn and anti products as in this case.
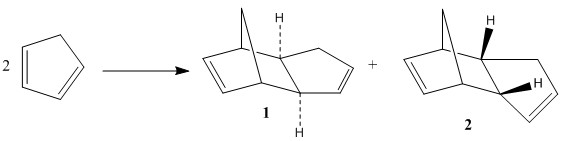
This reaction forms two stereoisomers the exo(molecule 1) and endo(molecule 2) conformers. The structures of both of these molecules have been optimized using molecular mechanics, based on the MMFF94s force field and using the conjugate gradient to populate the inverse Hessian matrix
Where G=gradient vector and H^{-1} = the inverse Hessian matrix. The conjugate gradient will only evaluate the diagonal terms setting the other terms to zero, this means it is slower but more accurate at less steep gradients.
| Energy/kcalmol^-1 | Molecule 1 | Molecule 2 |
|---|---|---|
| Bond Stretching | 3.54315 | 3.46833 |
| Angle Bending | 30.77260 | 33.18544 |
| Stretch Bending | -2.04142 | -2.08241 |
| Torsional | -2.73092 | -2.95125 |
| Out-of-Plane Bending | 0.01476 | 0.02186 |
| Van der Waals | 12.80158 | 12.36168 |
| Electrostatic | 13.01367 | 14.18714 |
| Total Energy | 55.37342 | 58.19079 |
This data shows that molecule 1 has a lower overall energy and therefore is the thermodynamic product and that molecule 2 is the kinetic product. The literature [3] states that it is the endo product that is formed exclusively at temperatures below 150°C and therefore in this case the higher energy dimer that is formed. This means that the reaction outcome is determined by the energy of the transition state and suggests the activation energy for the formation of the thermodynamic product (molecule 1) is high and therefore the comparable stability gotten from forming the exo product is not worth the extra energy needed to overcome this activation energy. The reasons for the differences in the energy of the endo and exo forms can be seen from their transition state diagrams.
|
|
Here it can be seen that the endo transition state will have more favorable interactions between non-bonding orbitals which aren't present in the exo. This is what leads to it being of lower energy and therefore explains why the activation energy for the endo product is lower and why it would be the favored product.
Hydrogenation of Dicyclopentadiene
The full hydrogenation of dicyclopentadiene occurs in two steps. The hydrogenation of the 6-membered ring occurs first and then the hydrogenation of the 5-membered ring.

This can be confirmed by comparing the energies of the two dihydrogenated intermediates to see which is of lower energy and therefore which hydrogenation is likely to occur. It would be predicted that molecule 4 would be of the lower energy as the literature [1] shows that it would be the first hydrogenation product. By using the same method as above the structures of molecules 3 and 4 can be optimised and their energies calculated.
| Molecule 3 | Molecule 4 | |
|---|---|---|
| Total Bond Stretching Energy/kcalmol^-1 | 3.31147 | 2.82315 |
| Total Angle Bending Energy/kcalmol^-1 | 31.93402 | 24.68555 |
| Total Stretch Bending Energy/kcalmol^-1 | -2.10215 | -1.65722 |
| Total Tortional Energy/kcalmol^-1 | -1.46923 | -1.65722 |
| Total Out-of-Plane Bending Energy/kcalmol^-1 | 0.01309 | 0.00028 |
| Total Van der Waals Energy/kcalmol^-1 | 13.63901 | 10.63714 |
| Total Electrostatic Energy/kcalmol^-1 | 5.11949 | 5.14702 |
| Total Energy/kcalmol^-1 | 50.44570 | 41.25748 |
This data shows that molecule 4 is of the lowest energy and that therefore it is the thermodynamic product and this reaction is thermodynamically controlled. By comparing the the relative contributions the the total energy by the different kinds of energy data available the major contribution to this difference can be found. The largest contributions to the energy of molecules 3 and 4 are the angle bending and van der Waals energy. The van der Waals energy suggests that there are more repulsive interactions within molecule 3 than 4. The difference in the angle bending energy shows there is more angle strain within molecule 3 this maybe due to the change in hybridization that occurs during hydrogenation. In going from sp2 to sp3 hybridized carbon the ideal bonding angle goes from 120° to 109°. In the case of molecule 3 this decrease in angle size is occurring in an already strained 5-membered ring rather than the less strained 6-membered ring of molecule 4 this maybe a reason that this change has a greater effect in molecule 3 and therefore that it's energy is higher.
Spectroscopic Simulation using Quantum Mechanics
Atropisomerism in an Intermediate Related to the Synthesis of Taxol

Molecules 9 and 10 are intermediates in the synthesis of Taxol. Atropisomerism results from rotation around a bond which has a high enough energy barrier to rotation that the two conformers can be isolated. These two molecules are examples of atropisomers and therefore by identifying the lowest energy conformer the stereochemistry of the carbonyl can be determined.
In this case the 6-membered ring of both conformers can be in either the chair or boat form as shown in the diagram. As there are two different ways the boat and chair can be arranged within the molecule there are therefore four possible conformers for each of these molecules. The chair conformation is of much lower energy than the boat or twist-boat conformations and so that would be expected to be much more stable[4].
| Molecule 9A - Chair | Molecule 9B - Boat | Molecule 9C - Chair | Molecule 9D - Boat | |
|---|---|---|---|---|
| Total Bond Stretching Energy/kcalmol^-1 | 8.62338 | 9.42123 | 7.57784 | 7.834097 |
| Total Angle Bending Energy/kcalmol^-1 | 33.41052 | 34.67988 | 21.64520 | 26.22237 |
| Total Stretch Bending Energy/kcalmol^-1 | 0.06903 | -0.02746 | 0.02511 | 0.15984 |
| Total Torsional Energy/kcalmol^-1 | 3.33127 | 8.81846 | -0.62531 | 5.09947 |
| Total Out-of-Plane Bending Energy/kcalmol^-1 | 1.46003 | 1.99612 | 0.93029 | 0.91805 |
| Total Van der Waals Energy/kcalmol^-1 | 35.91208 | 38.99023 | 33.52981 | 34.87548 |
| Total Electrostatic Energy/kcalmol^-1 | 0.22458 | 0.43125 | 0.28970 | 0.24658 |
| Total Energy/kcalmol^-1 | 83.03090 | 94.30970 | 63.37265 | 75.35677 |
| Molecule 10A - Chair | Molecule 10B - Boat | Molecule 10C - Chair | Molecule 10D - Boat | |
|---|---|---|---|---|
| Total Bond Stretching Energy/kcalmol^-1 | 7.59002 | 7.75314 | 8.47636 | 8.12911 |
| Total Angle Bending Energy/kcalmol^-1 | 18.80902 | 21.39287 | 30.18532 | 26.37625 |
| Total Stretch Bending Energy/kcalmol^-1 | -0.14014 | -0.06296 | -0.11104 | -0.02737 |
| Total Torsional Energy/kcalmol^-1 | 0.20473 | 4.51904 | 0.63579 | 1.08179 |
| Total Out-of-Plane Bending Energy/kcalmol^-1 | 0.84172 | 0.91527 | 1.51846 | 0.66608 |
| Total Van der Waals Energy/kcalmol^-1 | 33.30347 | 34.44053 | 34.92933 | 34.56470 |
| Total Electrostatic Energy/kcalmol^-1 | -0.05586 | -0.04036 | 0.31007 | -0.05834 |
| Total Energy/kcalmol^-1 | 60.55297 | 68.91753 | 75.94430 | 70.73221 |
As expected the chair conformation is lower in energy in both cases. Overall Molecule 9 is the more stable. By comparing the different energetic contributions we can see that the angle bending has a large contribution to the overall energy of both molecules. This suggests that the molecule is significantly strained as the lowest energy (zero) will occur when all the angles are at there optimum. The main contribution to angle strain in this case will be from the carbonyl. The carbonyl carbon will be sp2 hybridized and therefore will have an optimum angle of 120°. By looking at the break down of energies we can see that molecule 10 has a lower angle bending energy than molecule 9 which suggests that it has bond angles that are closer to the optimum. This is probably due to the conformation of the 6-membered ring, as the boat form has more eclipsed bonds than the chair form. There is also a close interaction between the two hydrogens at the ends of the boat. This will cause there to be more repulsive van der Waals interactions which is the reason that both the van der Waals and tortional energy contributions for the boat conformation are higher than those for the boat conformer[4]
Spectroscopy of an Intermediate Related to the Synthesis of Taxol
By first optimizing the energy of a molecule then running a calculation using a B3LYP 6-31G(d,p) basis set, NMR spectra of that molecule can be predicted. The accuracy of, firstly, the energy optimization and secondly, the predicted NMR spectra can be tested by comparison of the shifts to the literature values.
Two derivatives of molecules 9 and 10, 17 and 18 are also intermediates in the formation of Taxol. After the optimization shown below and identification of the lowest energy conformer of each molecule the 13C and 1H NMR Spectra of both molecules was predicted using the Gaussian Software. It is hoped that if the molecule had been fully optimized the predicted spectra will show a very close resemblance to that found in the literature.
Molecule 17
| 17A - Boat | 17B - Chair | 17C - Chair | 17D - Boat | |
|---|---|---|---|---|
| Total Bond Stretching Energy/kcalmol^-1 | 14.43192 | 14.22872 | 14.58796 | 15.52154 |
| Total Angle Bending Energy/kcalmol^-1 | 30.51234 | 30.40146 | 34.82206 | 39.20442 |
| Total Stretch Bending Energy/kcalmol^-1 | -0.03909 | 0.01771 | 0.34390 | 0.02885 |
| Total Torsional Energy/kcalmol^-1 | 15.75087 | 13.88775 | 11.55837 | 13.06256 |
| Total Out-of-Plane Bending Energy/kcalmol^-1 | 1.29290 | 1.18156 | 0.54495 | 0.38659 |
| Total Van der Waals Energy/kcalmol^-1 | 52.70154 | 50.70397 | 51.05059 | 54.15917 |
| Total Electrostatic Energy/kcalmol^-1 | -6.64141 | -6.51626 | -7.69687 | - 6.65699 |
| Total Energy/kcalmol^-1 | 108.00907 | 103.90490 | 105.21098 | 115.70615 |
Here, as expected, the lowest energy conformation is a chair conformation. The difference between the two chair conformers is small, the main differences can be seen in the angle bending energy and the torsional energy suggesting that the bonds within the molecule are more strained in the 7C as compared to the 7B.
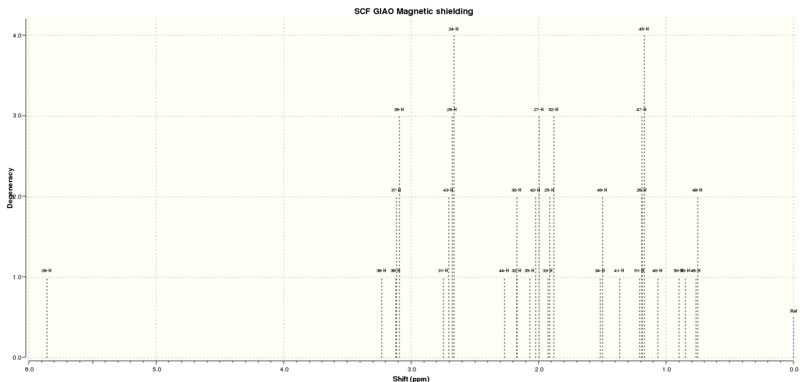
1H NMR of Molecule 17[5]
| Predicted Shift (CDCl3)(ppm) | Atom Number | Literature (300MHz, CDCl3) Shift[6] (ppm) | Multiplicity | Integration |
|---|---|---|---|---|
| 5.86 | 28 | 4.84 | dd, J=7.2 and 4.7Hz | 1H |
| 3.23 | 38 | 3.40-3.10 | m | 4H |
| 3.11 | 36,37,39 | 2.99 | dd, J=6.8 and 5.2Hz | 1H |
| 2.70 | 31,43,29,24 | 2.80-1.35 | m | 14H |
| 2.27 | 44 | |||
| 2.17 | 30,32 | |||
| 2.03 | 27,35,42 | |||
| 1.91 | 25,33,52 | |||
| 1.51 | 34,49 | |||
| 1.36 | 41 | 1.38 | s | 3H |
| 1.19 | 26,45,47,51 | 1.25 | s | 3H |
| 1.06 | 40 | 1.10 | s | 3H |
| 0.90 | 50 | 1-0.8 | m | 1H |
| 0.85 | 53 | |||
| 0.76 | 46,48 |
Overall this spectrum is reasonably accurate, with quite good general matches to the literature, however there are some differences in relative intensities. This can be somewhat explained by the fact that the program does not take the heavy atom effect into account when calculating values for sulphur and so they are not as accurate, and it is mainly for the atoms near to the sulphurthat the discrepancies between literature and prediction exist. The highest shift in the predicted spectra is quite a lot higher than that in the literature. The expected multiplet between 3.40 and 3.10 is distinguishable within the predicted spectra. There is a well matched peak at 1.36 (predicted) and 1.38 (Literature). There is a possibility that the literature shift at 2.99ppm is seen at a much lower shift in the predicted spectrum. By looking at the expected and predicted integration values the 2.99 shift could be seen at 0.85-0.76 instead however there is no obvious reason why these atoms should be so different to their expected values.
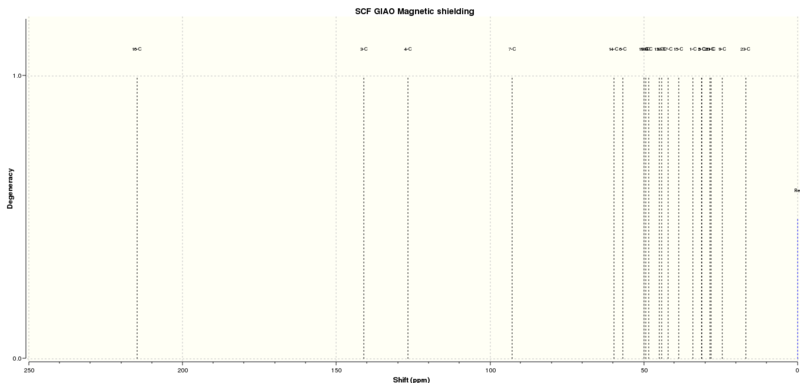
13C NMR of Molecule 17[5]
| Predicted Shift (ppm) | Atom Number | Literature (75MHz, CDCl3) Shift[6] (ppm) |
|---|---|---|
| 214.70 | 16 | 218.79 |
| 141.10 | 3 | 144.63 |
| 126.71 | 4 | 125.33 |
| 92.86 | 7 | 72.88 |
| 59.65 | 14 | 56.19 |
| 56.80 | 6 | 52.52 |
| 50.00 | 19 | 48.50 |
| 49.48 | 18 | 46.80 |
| 48.36 | 8 | 45.76 |
| 45.00 | 13 | 39.80 |
| 44.15 | 12 | 38.81 |
| 42.06 | 17 | 35.85 |
| 38.59 | 15 | 32.66 |
| 34.07 | 1 | 28.29 |
| 31.28 | 2 | 26.88 |
| 31.23 | 5 | 25.66 |
| 28.55 | 20 | 23.86 |
| 28.23 | 21 | 20.96 |
| 24.59 | 9 | 18.71 |
| 16.77 | 23 |
The carbon NMR Spectrum is also quite well matched however there are some anomalies that have to be discussed. The largest 3 shifts are very similar in both predicted and literature values however then there is a anomaly in atom 7. This is due to it being the carbon connected to both sulphurs and as discussed before the program does not compute sulphur well, and this is actually more likely to actually be at a lower shift. There are three anomalous shifts that don't match up in both spectra (predicted: 31.23, 48.36, 59.65, Literature:20.96 ,25.66 ,72.88ppm), these all relate to carbons near to the sulphur atoms which goes someway to explain why they are anomalous. This backs up the idea that the program used has a poor ability in computing sulphur.
Molecule 18
| 18A - Boat | 18B - Chair | 18C - Chair | 18D - Boat | |
|---|---|---|---|---|
| Total Bond Stretching Energy/kcalmol^-1 | 14.01844 | 13.55684 | 13.70514 | 13.91526 |
| Total Angle Bending Energy/kcalmol^-1 | 28.42921 | 29.38731 | 30.65749 | 32.43784 |
| Total Stretch Bending Energy/kcalmol^-1 | 0.05114 | 0.01685 | 0.35880 | 0.35074 |
| Total Tortional Energy/kcalmol^-1 | 13.43099 | 11.78522 | 9.20335 | 13.38296 |
| Total Out-of-Plane Bending Energy/kcalmol^-1 | 0.76685 | 0.76743 | 1.01427 | 0.88202 |
| Total Van der Waals Energy/kcalmol^-1 | 50.65015 | 48.37074 | 48.56712 | 50.83510 |
| Total Electrostatic Energy/kcalmol^-1 | -6.64451 | -6.91913 | -6.88914 | -7.28433 |
| Total Energy/kcalmol^-1 | 100.70226 | 96.96526 | 96.61704 | 104.51959 |
Here we can see that again the chair conformations are the lowest energy conformers and also that they are close together in energy. These conformers again show the largest difference in angle bending and tortional energy but in this case it is the 18C that is slightly lower in energy however as the energies are so close the correct conformation can't be known with exact certainty, however it is assumed that the 18C is the lowest and so it is that conformer that will be studied further.
1H NMR of Molecule 18[7]
| Predicted Shift (CDCl3)(ppm) | Atom Number | Literature (300MHz, C6D6) Shift[6] (ppm) | Multiplicity | Integration |
|---|---|---|---|---|
| 5.38 | 28 | 5.21 | m | 1H |
| 3.27 | 31, 39 | 3.00-2.70 | m | 6H |
| 3.16 | 38 | |||
| 3.07 | 30, 37 | |||
| 2.96 | 36, 42 | |||
| 2.62 | 26 | 2.70-2.35 | m | 4H |
| 2.54 | 24 | |||
| 2.39 | 29 | |||
| 2.21 | 32 | 2.20-1.70 | m | 6H |
| 2.02 | 33, 44, 47 | |||
| 1.93 | 34 | |||
| 1.82 | 27 | |||
| 1.56 | 25, 35, 40, 43, 45, 49 | 1.58 | t (J=5.4Hz) | 1H |
| 1.41 | 52 | 1.50-1.20 | m | 3H |
| 1.03 | 41, 46, 50, 53 | 1.10 | s | 3H |
| 0.81 | 48 | 1.07 | s | 3H |
| 0.72 | 51 | 1.03 | s | 3H |
Spectra taken in benzene was found to cause the frequency of the applied field to be reduced by 1.13ppm in methane gas at a fixed frequency[8]. This means that the literature referred to here could be lower in shift than the predicted spectrum due to solvent effects. If this is taken into account, the predicted spectrum does show a good match to the literature values. There is some overlap between the 3.00-2.70 and 2.70-2.35 multiplets in the predicted spectrum as well as in the 1.50-1.20 range, but the rest of the shift values match well with the literature and so this is overall an accurate prediction of this spectrum.

13C NMR of Molecule 18[7]
| Predicted Shift CDCl3(ppm) | Atom Number | Literature (75MHz, C6D6) Shift[6] (ppm) |
|---|---|---|
| 209.44 | 16 | 211.49 |
| 150.78 | 3 | 148.72 |
| 122.70 | 4 | 120.90 |
| 89.90 | 7 | 74.61 |
| 61.71 | 6 | 60.53 |
| 58.49 | 14 | 51.30 |
| 54.25 | 18 | 50.94 |
| 49.86 | 19 | 45.53 |
| 47.47 | 8 | 43.28 |
| 46.60 | 13 | 40.82 |
| 44.31 | 12 | 38.73 |
| 38.08 | 17 | 36.78 |
| 36.92 | 15 | 35.47 |
| 35.48 | 1 | 30.84 |
| 28.49 | 21 | 30.00 |
| 28.42 | 5 | 25.56 |
| 25.28 | 2 | 25.35 |
| 24.99 | 9 | 22.21 |
| 23.21 | 20 | 21.39 |
| 17.74 | 23 | 19.83 |
This predicted spectrum shows a very similar relationship to the literature as for molecule 17. Similarly to the previous cases there are three peaks that are anomalous in both spectra (predicted:47.74,61.71,89.90, Literature:21.39,40.82,74.61) which relate to atoms that are neighbours or near-neighbours to the sulphur atoms. The rest of the shifts are pretty accurate to their expected values especially given the effects of differing solvents between the two spectra discussed above.
Analysis of the Properties of the Synthesised Alkene Epoxides
The Shi Asymmetric Fructose Catalyst
The Shi Catalyst is used to catalyse the epoxidation of trans alkenes as shown below.
The Shi catalyst is derived from carbohydrates, specifically fructose. It can be used to ensure high enantioselectivity in the epoxidation of di and trisubstituted alkenes[10]. 21 is a pre-catalyst of this reaction from which the catalyst is produced by a reaction with persulfuric acid. By using the cambridge crystal database(CCDC) and its conquest software, the crystal structure of molecule 21 can be found. Using this software the CO bond lengths surrounding each anomeric center can be determined and analysed. The anomeric centers in this molecule are between O1-C7-O2, O2-C2-O6 and O5-C10-O4 The atom labels used in this discussion refer to the molecule's thumbnail on the right.
| Carbon Atom (A) | Oxygen Atom (A) | Bond Length |
|---|---|---|
| O3 (Carbonyl) | 1.197 | |
| C7 | O1 | 1.413 |
| O2 | 1.441 | |
| C2 | O2 | 1.403 |
| O6 | 1.403 | |
| C10 | O4 | 1.439 |
| O5 | 1.409 |
Firstly comparison of the carbonyl and CO single bond lengths is a good confirmation of the double-bond character of the carbonyl, as it is 1.197A as compared to around 1.4A for the CO single bonds. For the three anomeric centers only one has equal bond lengths on both sides of the carbon atom, the anomeric center around C2. This maybe because this center isn't part of a ring and therefore there is not as much strain on the bond angles. When measuring the bond angles the O2-C2-O6 bond angle is 113.03° as compared to the O4-C10-O5 105.60°and the O1-C7-O2 104.40° which is more strained compared to the optimum sp3 bond angle of 109°. This bond angle strain may also be the reason that the O4-C10-O5 center shows slightly shorter bonds in comparison to the O1-C7-O2 center. In the cases of both of these centers one length is longer than the other, this maybe due to the proximity of the the individual oxygens to the carbonyl group and therefore the interaction between the two oxygens.
The Jacobsen Asymmetric Catalyst
The Jacobsen Catalyst uses a schiff base Mn complex to achieve asymmetric epoxidation. Its formation is shown in the scheme below
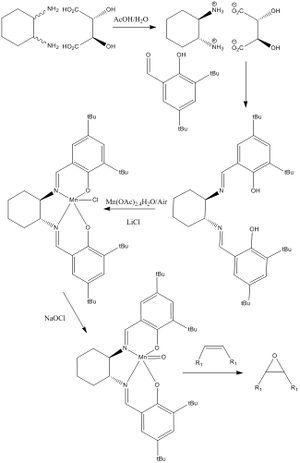
The alkene will approach the catalyst from the top face directly onto the oxygen ligand as the other approached are blocked by the bulky groups. This can be proven by looking at the distances between the tBu groups which block the approach from the front face. The closeness of the tBu groups shown in the image on the right prevents approach from the alkene. The van der Waals radius for carbon atoms is around 1.7A[12] so the distance between the two tBu is approaching a bond length, there may even be some kind of non-covalent interaction between them as discussed below.
Epoxide Synthesis
Styrene Epoxide
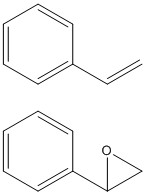
Styrene is the monomer of polystyrene which is a cheap and hard plastic whose uses including packing materials. Styrene can be epoxidised using both catalysts discussed above producing styrene oxide. In this study the optimised structure of styrene oxide as well as it's NMR spectra, optical rotation and transition states with both catalysts are investigated using the same techniques as previously.
| Styrene Oxide | |
|---|---|
| Total Bond Stretching Energy/kcalmol^-1 | 1.84193 |
| Total Angle Bending Energy/kcalmol^-1 | 1.42461 |
| Total Stretch Bending Energy/kcalmol^-1 | -0.74663 |
| Total Torsional Energy/kcalmol^-1 | 2.34618 |
| Total Out-of-Plane Bending Energy/kcalmol^-1 | 0.00163 |
| Total Van der Waals Energy/kcalmol^-1 | 13.67464 |
| Total Electrostatic Energy/kcalmol^-1 | 3.07272 |
| Total Energy/kcalmol^-1 | 21.61508 |
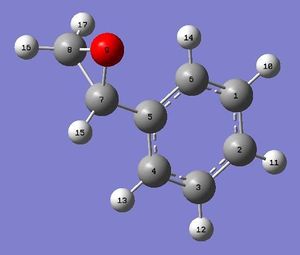
Here the lowest energy conformation of styrene oxide is that where the phenyl ring is perpendicular to the epoxide. This will minimise unfavorable interactions between the hydrogens on the benzene ring and the epoxide by moving them further away from each other in space.
H1 NMR of Styrene Epoxide [13]
| Predicted Shift (CDCl3) (ppm) | Atom Number | Literature (300MHz, CDCl3) Shift[14] (ppm) | Multiplicity | Integration |
|---|---|---|---|---|
| 7.49 | 13, 10, 12, 11 | 7.1-7.4 | m | 5H |
| 7.30 | 14 | |||
| 3.66 | 15 | 3.6 | dd | 1H |
| 3.12 | 15 | 3.1 | dd | 1H |
| 2.54 | 17 | 2.8 | dd | 1H |
The 1H NMR spectrum of styrene oxide has a very good correlation with the literature spectrum. The only small difference is the lowest shift is a little higher than expected in the predicted spectrum, but this could be due to its proximity to the oxygen of the epoxide.

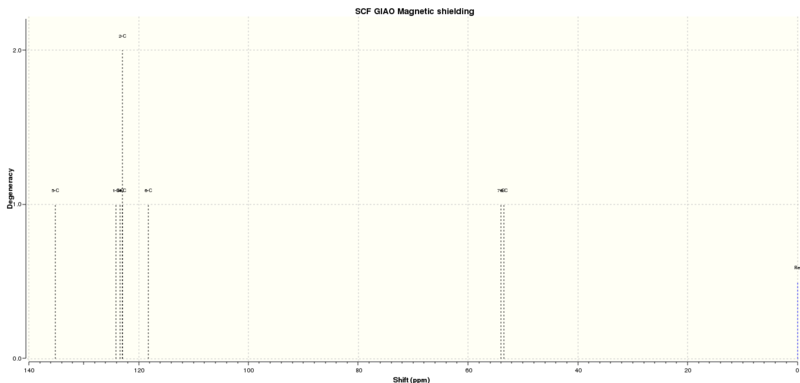
13C NMR of Styrene Epoxide [13]
| Predicted Shift (CDCl3) (ppm) | Atom Number | Literature (75MHz, CDCl3) Shift [15](ppm) |
|---|---|---|
| 135.13 | 5 | 137.5 |
| 124.13 | 1 | 128.2 |
| 123.41 | 3 | 128.0 |
| 122.96 | 4, 2 | 125.3 |
| 118.27 | 6 | |
| 54.06 | 7 | 51.1 |
| 53.47 | 8 | 51.0 |
The 13C spectrum is a little less well matched, the higher shifts are all at a lower ppm values than expected from the literature and so it is hard to assign which shifts match up to each other. At the lower shifts the ppm values are higher than expected in the predicted spectrum. However overall the shifts that are expected are seen and so this is a reasonable prediction of the spectrum.
β-methyl styrene
β-methyl styrene is closely related to styrene, it can also be epoxidised by both the Shi and Jacobsen catalyst. The oxide can have two different forms trans and cis, their relative energies are shown below.
| trans-β-methyl Styrene Oxide | cis-β-methyl Styrene Oxide | |
|---|---|---|
| Total Bond Stretching Energy/kcalmol^-1 | 1.88638 | 1.92546 |
| Total Angle Bending Energy/kcalmol^-1 | 1.73667 | 2.17295 |
| Total Stretch Bending Energy/kcalmol^-1 | -0.76428 | -0.70673 |
| Total Torsional Energy/kcalmol^-1 | 2.89543 | 2.91445 |
| Total Out-of-Plane Bending Energy/kcalmol^-1 | 0.00161 | 0.00194 |
| Total Van der Waals Energy/kcalmol^-1 | 14.32595 | 14.54294 |
| Total Electrostatic Energy/kcalmol^-1 | 3.03856 | 3.03849 |
| Total Energy/kcalmol^-1 | 23.12031 | 23.88950 |
As expected the trans form is of lower energy as there are less steric clashes between the bulky substituents, as they are far apart in space.
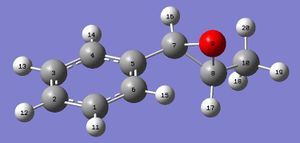
1H NMR of trans-β-methyl styrene oxide [16]
| Predicted Shift (ppm) | Atom Number | Literature (400MHz, CDCl3) Shift[17] (ppm) | Multiplicity | Integration |
|---|---|---|---|---|
| 7.49 | 11, 13, 14 | 7.24-7.39 | m | 5H |
| 7.42 | 12 | |||
| 7.31 | 15 | |||
| 3.41 | 16 | 3.57 | d J=4.3Hz | 1H |
| 2.79 | 17 | 3.32-3.40 | m | 1H |
| 1.68 | 19 | 1.45 | d j=5.4Hz | 3H |
| 1.59 | 18 | |||
| 0.72 | 20 |
The predicted 1H NMR spectrum of trans-β-methyl styrene is relatively accurate compared to the literature. There is some discord in the lower shifts. For example the shift expected at 1.45 is more spread out in the predicted spectrum and the shift at 3.57 is lower than expected. These discrepancies may be because these atoms are the ones that are closest to the epoxide and so the oxygen may have some effect on their ppm values not taken into account by the program when calculating the shifts.
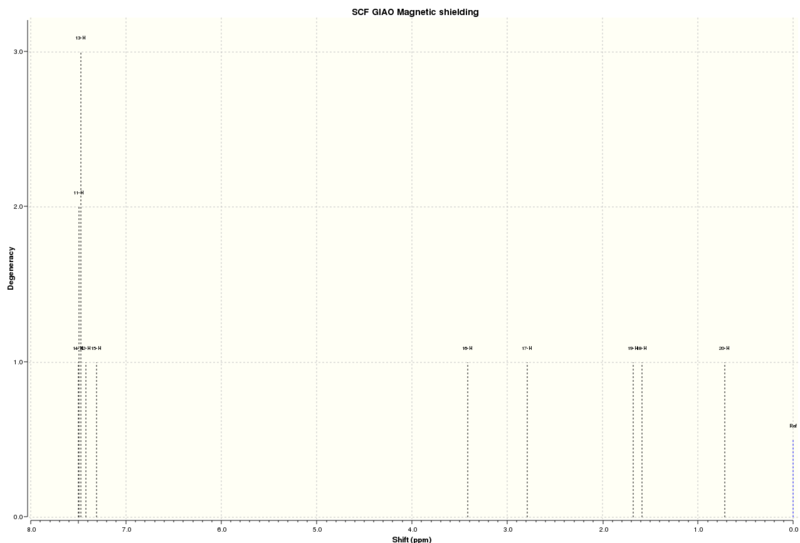
13C NMR of trans-β-methyl styrene oxide[16]
| Predicted Shift (ppm) | Atom Number | Literature (100MHz, CDCl3) Shift [18](ppm) |
|---|---|---|
| 134.98 | 5 | 128.3 |
| 124.07 | 1 | 127.7 |
| 123.33 | 3 | 125.7 |
| 122.80 | 4 | |
| 122.73 | 2 | |
| 118.49 | 6 | |
| 62.32 | 8 | 59.7 |
| 60.58 | 7 | 59.7 |
| 18.84 | 10 | 18.1 |
There are more obvious differences between the predicted and literature 13C spectra. This maybe due to the way the NMR data is reported in the literature as each shift could refer to more than one carbon and without the full assignments it can't be known which carbon the authors meant to refer to. However the shifts that can be matched up are a reasonable match, especially at the lower shifts, and you would expect that in the literature spectrum the peaks around 122 would all be seen as a single shift and so recorded as such.
Absolute Configurations of the Epoxides
The Optical rotation of the epoxides can be computed at any wavelength in this case we are looking at the rotation at 589 and 365nm. 589nm is yellow light emitted by an excited sodium atom, for example in a sodium light, and 365nm which is the wavelength for Blacklight blue lamps. As 589nm is a much more common wavelength there is a lot of literature covering the optical rotation at this wavelength but there is very little data at 365nm and therefore I have assumed a similar level of accuracy for values at both wavelengths by only comparing the values at 589nm.
For the styrene epoxide optical rotation was first measured at -184.28degrees(589nm) and -694.75(365nm) [19] it was quickly decided that these did not match up with the literature values and so other conformations of the molecule, where the phenyl ring was arranged differently as compared to the epoxide group, were explored. This eventually lead to optical rotations of -30.42deg.(589nm) and -94.99deg.(365nm)[20] this is very close to the literature value and therefore it can be assumed that this was the conformer that had been produced in the literature. Interestingly the conformer with the closer optical rotation to the literature value is that of higher energy. This may suggest that it is not the most thermodynamically stable conformer and that the epoxidation reaction is more likely to be under kinetic rather than thermodynamic control.
| Conformer | Total Energy (kcal/mol) | Optical Rotation [Alpha] (degrees) | Literature Optical Roatation values [Alpha] 589nm (degrees)[21] | |
|---|---|---|---|---|
| 589nm | 365nm | |||
| 1 | 21.61507 | -184.28 | -694.75 | -33.3 |
| 2 | 24.54061 | -30.42 | -94.99 | -33.3 |
The literature and predicted values for trans-β-methyl styrene the on the other hand, differ quite substantially. The difference between the two values for suggests that this also has two conformers with different optical rotation values, however despite attempts the other conformer couldn't be found and therefore a calculation of its optical rotation wasn't explored to confirm this.
| Total Energy (kcal/mol) | Optical Rotation [Alpha] (degrees)[22] | Literature Optical Rotation values [Alpha] 589nm (degrees) | |
|---|---|---|---|
| 589nm | 365nm | ||
| 23.12031 | -103.36 | -435.64 | 66[23] |
Transition States
β-Methyl Styrene
The enantiomeric excess for the transition states using the two catalysts discussed above can be found for β-methyl styrene. By using the output files of the pre-run calculations[24], which would take too long to run ourselves due to the time constraints of this experiment, the conformation of the transition state with the lowest free energy for each can be found. By using the equation,
this information can be used to find the ratio of concentrations between the two diastereomeric transition states(K). Then this K value can be used to calculate the value of enantiomeric excess of one epoxide over the other as a percentage.
Shi Catalyst
The lowest energy transition states in the case of the Shi catalyst are set 4, where [R,R] = -1343.032443 and [S,S] = -1343.024742 Hartrees. By using the equation above both the K and ee values can be calculated for this each variation of the transition state.
| Shi | ||||||
|---|---|---|---|---|---|---|
| TS | Sum of electronic and thermal Free Energies | ΔG | K | ee | ||
| R,R | S,S | Hartree | J/mol | |||
| 1 | -1343.022970 | -1343.017942 | 0.005028 | 13201.015 | 0.004865792 | 0.990316 |
| 2 | -1343.019233 | -1343.017942 | 0.00363 | 9530.5657 | 0.021390662 | 0.958115 |
| 3 | -1343.029272 | -1343.023766 | 0.005506 | 14456.0041 | 0.002932761 | 0.994152 |
| 4 | -1343.032443 | -1343.024742 | 0.007701 | 20218.977 | 0.000286808 | 0.999427 |
This means that the ee value for set 4 is 99.94% which suggests that the [R,R] enantiomer is much favored over the [S,S] transition state.
Jacobsen Catalyst
In this case we choose the lowest energy transition states for cis and trans β-methyl styrene (set 1 in each case) to study the ee values of. These values were calculated in the same way as above.
| Jacobsen - cis-β-Methyl Styrene | ||||||
|---|---|---|---|---|---|---|
| TS | Sum of electronic and thermal Free Energies | ΔG | K | ee | ||
| S,R | R,S | Hartree | J/mol | |||
| 1 | -3383.259559 | -3383.25106 | 0.008499 | 22314.1262 | 0.000123173 | 0.999754 |
| 2 | -3383.253442 | -3383.25027 | 0.003172 | 8328.08663 | 0.034745708 | 0.932842 |
The ee value for the lowest energy S,R and R,S transition states (set 1) is 99.98% this suggests that the S,R enantiomer is the most favored.
| Jacobsen - Trans-β-Methyl Styrene | ||||||
|---|---|---|---|---|---|---|
| TS | Sum of electronic and thermal Free Energies | ΔG | K | ee | ||
| S,S | R,R | Hartree | J/mol | |||
| 1 | -3383.262481 | -3383.253816 | 0.008665 | 22749.9592 | 0.000103314 | 0.999793 |
| 2 | -3383.257847 | -3383.254344 | 0.003503 | 9197.1272 | 0.02447053 | 0.952228 |
The ee value for the trans-β-methyl styrene (considering set 1 also) is also 99.98% suggesting the S,S enantiomer is almost exclusively preferred.
The ee value for the trans-β-methyl styrene is only slightly higher than for the cis isomer and therefore there S,S in the trans is not hugely preferred compared to S,R in the cis. This closeness in free energy value shows the same trend as found previously for the stabilisation of β-methyl styrene, where the trans was favored but only by a small amount over the cis.
NCI
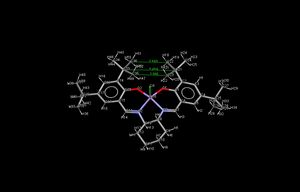
The electron density seen in non-covalent interactions(NCI), such as hydrogen bonds and electrostatic attractions, can be shown through a image showing the different levels of attractiveness within the molecule. This can be used to explain the NCI contributions to the energy of a transition state.
Here the transition state for the Jacobsen epoxidation of styrene[25] has been studied, specifically the second transition state in the S series. The different colors within the surface below correspond to different strengths of attraction: Blue is very attractive, green is mildly attractive, yellow is mildly repulsive and red is very repulsive.
Orbital |
From this image we can see where the attractive interactions are within the molecule and hence where the inherent stability of the transition state comes from. The first important interaction to notice is that between the two tBu group on the catalyst molecule. This is mildly attractive and helps to act as a conformational lock as well as to prevent the approach of the alkene from that side, as discussed above. The second is the attractive interactions between the phenol ring of the styrene and the catalyst. These two rings are stacked which helps promote electrostatic interactions between the two rings, much like base stacking in DNA, this can clearly been seen in the NCI. Finally there is repulsive interactions between the oxygen and other Mn ligands, but also between the oxygen and styrene molecule. Such interactions, known as half-covalent interactions, show the transition state in preparation to transfer the oxygen to the styrene.
QTAIM

QTAIM is used to investigate the electronic topology of the active-site in the transition state. It shows the electron density that can be found between two atoms, and defines the bond critical point (BCP) within a molecule. The BCP show where there is the possibility of a bond being formed. The program puts a solid line between atoms where it is sure a bond will exist and only a dotted line where there is some form of non-covalent interaction between the atoms.
By looking at the same transition state as for the NCI analysis above, the second Jacobsen styrene S series[26], the image above is produced. The interactions shown between the styrene molecule and the catalyst control act as a conformational lock to he transition state. You can also see from this image the interaction between the two tBu groups in the catalyst, these interaction provide stabilization to the molecule lowering the energy of the transition state.
Other Possible Candidates for Study
Limonene Oxide is a small epoxide with a molecular weight of 152.24. It can be produced from the equivalent alkene as shown below[27] producing a racemic mixture of products. The suggested reaction conditions in this case are mPCBA, CHCl3, ), 0°C, 2Hrs leading to a 62% yield.
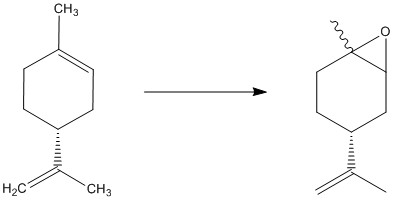
By optimizing the energy in the same way as above, two of the conformers of limonene oxide, energies and literature optical rotations can be compared.
| Conformer | Total Energy (kcal/mol) | Optical Rotation, 589nm (degrees) |
|---|---|---|
| 1 | 32.18281 | 76[28] |
| 2 | 29.66874 | 44[29] |
It would be interesting to discover if the literature optical rotations for these conformers and others are accurate as there is some disagreement between papers in the literature as to a definate value. This molecule also has medical interest in the treatment of mange infections caused by insects as well as other skin conditions.
References
- ↑ 1.0 1.1 1.2 1.3 D. Skala, J. Hanika, Petroleum and Coal, 2003, 45, 106-8.
- ↑ [1] Henry S. Rzepa, Imperial College, Organic Pericyclic Reactions: Categories
- ↑ J. Krupka, Petroleum & Coal,2010, 52(4), 290-306.
- ↑ 4.0 4.1 4.2 R. F. Daley, S. J. Daley, Organic Chemistry - Ch 3,2008, 143
- ↑ 5.0 5.1 Bethany Evans - D-space DOI:10042/27189
- ↑ 6.0 6.1 6.2 6.3 L. A. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. Rogers, J. Am. Chem. Soc.,1990, 112 (1), 277-83
- ↑ 7.0 7.1 Bethany Evans - D-space DOI:10042/27188
- ↑ A. D. Buckingham, T. Schaefer, W. G. Schneider, J. Chem. Phys.,1960, 32 (4), 1227-33
- ↑ A. Burke, P. Dillon, K. Martin, T. W. Hanks, J. Chem. Educ., 2000, 77 (2), 271.
- ↑ Z.-X. Wang , Y. Tu , M. Frohn , J.-R. Zhang , and Y. Shi, J. Am. Chem. Soc., 1997, 119 (46), 11224-11235.
- ↑ J. Hanson, J. Chem. Educ.,2001, 78 (9), 1266.
- ↑ A. Bondi, J. Phys. Chem.,1964, 68 (3), 441-51.
- ↑ 13.0 13.1 Bethany Evans - D-space DOI:10042/27186
- ↑ J. Adhikary, A. Guha, T. Chattopadhyay, D. Das, Inorganica Chimica Acta, 2013, 406, 1-9.
- ↑ C. Wiles, M. J. Hammond, P. Watts, Beilstein Journal of Organic Chemistry, 2009, 5(27), 1-12 .
- ↑ 16.0 16.1 Bethany Evans - D-space DOI:10042/27343
- ↑ P. C. Bulman Page, P. Parker, B. R. Buckley, G. A. Rassias, D. Bethell,Tetrahedron, 2009, 65, 2910-5.
- ↑ S. A. Kavanagh, A. Piccinini, S. J. Connon,Advanced Synthesis & Catalysis,2010, 352 (11-2), 2089-93.
- ↑ Bethany Evans - D-space DOI:10042/27195
- ↑ Bethany Evans - D-space DOI:10042/27185
- ↑ F. R. Jensen, R. C. Kiskis, J. Am. Chem. Soc., 1975, 97(20), 5825-31.
- ↑ Bethany Evans - D-space DOI:10042/27359
- ↑ Rabe, Chemische Berichte,1911,44, 826.
- ↑ [2] Henry S. Rzepa, Imperial College, 1C: Structure modelling, NMR and Chiroptical simulations
- ↑ [3] Henry S. Rzepa, Imperial College, 1C: Structure modelling, NMR and Chiroptical simulations
- ↑ [4] Henry S. Rzepa, Imperial College, 1C: Structure modelling, NMR and Chiroptical simulations
- ↑ S. M. Wilkinson, J. Price, M. Kassiou, Tetrahedron Letters, 2013, 54 (1), 52-4.
- ↑ Z-B. Xu, J. Qu, Chinese Journal of Chemistry, 2012, 30(5), 1133-6.
- ↑ D. Steiner, L. Ivison, C. T. Goralski, R. B. Appell, J. R. Gojkovic, B. Singaram , Tetrahedron: Asymmetry, 2002, 13(21), 2359-63.
