Rep:Mod:BB12321
Benjamin Bryant Inorganic Labs 01/05-05/05
EX3
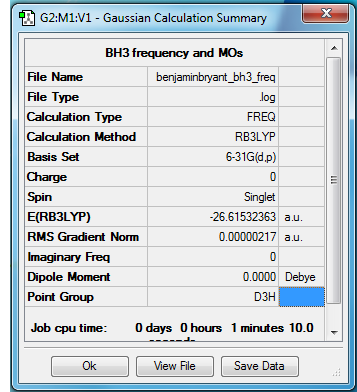
BH3
Method - RB3LYP
Basis set - 6-31G(d.p)
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000002 0.000300 YES Maximum Displacement 0.000017 0.001800 YES RMS Displacement 0.000009 0.001200 YES Predicted change in Energy=-1.107906D-10 Optimization completed.
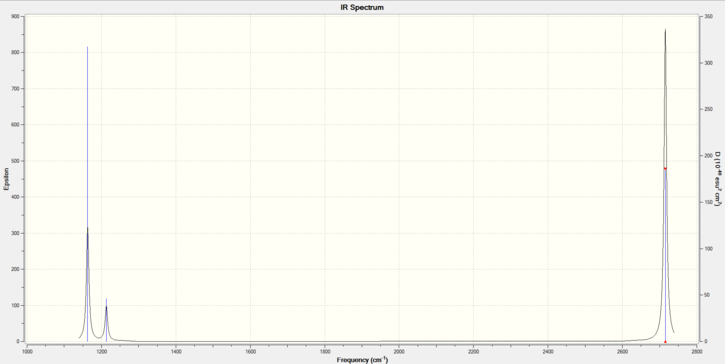
Low frequencies --- -0.6934 -0.4033 -0.0054 12.2717 15.9847 15.9997 Low frequencies --- 1163.0341 1213.2080 1213.2107
test molecule |
File:BENJAMINBRYANT BH3 FREQ.LOG
| Wavenumber | Intensity | Symmetry | IR Active | Type |
|---|---|---|---|---|
| 1163 | 93 | A2 | Yes | Out of plane bend |
| 1213 | 14 | E' | Very slight | Bend |
| 1213 | 14 | E' | Very slight | Bend |
| 2582 | 0 | A1' | No | Symmetric stretch |
| 2715 | 126 | E' | Yes | Asymmetric stretch |
| 2715 | 126 | E' | Yes | Asymmetric stretch |
There are less than six peaks in the spectrum due to the fact that some of the vibrations are degenerate and also due to the fact that for one vibration has an intensity of zero. (as shown by the infrared value).
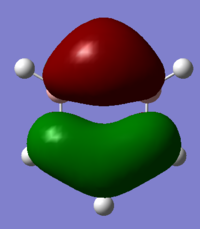
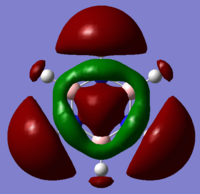
The diagram above shows the LCAO predicted from a qualitative MO diagram and using Gaussview. In the drawn MO diagram, the position of the atomic orbitals in the predicted molecular orbitals is shown. In what is predicted by Gaussview, the overlap of LCAO is shown. There are no significant differences between the ordering of MOs in real and LCAO, however in more complex molecules, the use of the qualitative method is difficult - especially when mixing is involved. This means the method is reasonably accurate up until a point.
Smf115 (talk) 07:22, 17 May 2018 (BST)Clear inclusion of the MOs in the diagrama and a good point about the extent to which the theory is accurate.
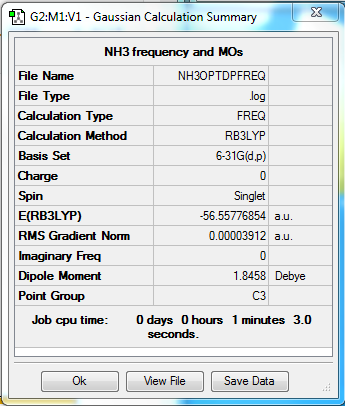
NH3
Method - RB3LYP
Basis set - 6-31G(d.p)
Item Value Threshold Converged? Maximum Force 0.000088 0.000450 YES RMS Force 0.000032 0.000300 YES Maximum Displacement 0.000361 0.001800 YES RMS Displacement 0.000194 0.001200 YES Predicted change in Energy=-5.649360D-08 Optimization completed.
Low frequencies --- -32.4235 -32.4224 -11.4276 -0.0044 0.0115 0.0476 Low frequencies --- 1088.7628 1694.0251 1694.0251
test molecule |
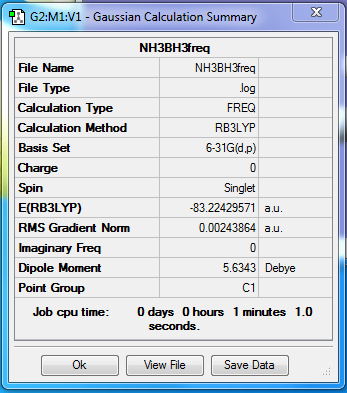
NH3BH3
Method - RB3LYP
Basis set - 6-31G(d.p)
Item Value Threshold Converged? Maximum Force 0.000137 0.000450 YES RMS Force 0.000038 0.000300 YES Maximum Displacement 0.001016 0.001800 YES RMS Displacement 0.000225 0.001200 YES Predicted change in Energy=-1.126084D-07 Optimization completed.
Low frequencies --- -170.0059 -107.9511 -105.1311 -0.0007 -0.0004 0.0006 Low frequencies --- 220.9752 634.4913 635.9012
test molecule |
Smf115 (talk) 22:08, 15 May 2018 (BST)Included all the necessary structure information but it should have been noted that the low frequencies here are very high! If you check your log file you can see the calculation hasn't converged and so it should have been recalculated.
Energy Analysis
E(NH3)= -56.55776871 A.U
E(BH3)= -26.61532363 A.U
E(NH3BH3)= -83.22429571 A.U
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
In A.U, dissociation energy = -0.05120338 A.U
In kJ/mol, dissociation energy = -134.4088725 kJ/mol
From these calculations it can be said that the C-N bond is weak dative, this is from comparison with a normal C-C covalent bond which is approximately -300 kJ/mol.
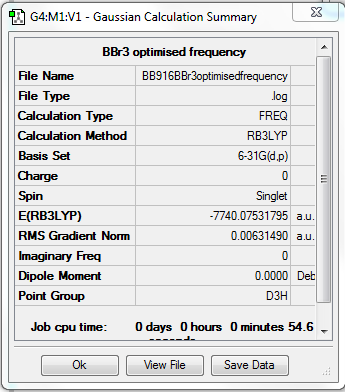
BBr3
Method - B3LYP
Basis set - 6-31G(d,p)
Pseudo potential basis set - LANL2DZ
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000023 0.001200 YES Predicted change in Energy=-4.027498D-10 Optimization completed.
Low frequencies --- -0.0077 -0.0074 0.0113 33.9545 34.1451 34.1451 Low frequencies --- 148.3906 148.3924 258.5061
test molecule |
File:BB916BBr3optimisedfrequency.log
http://hdl.handle.net/10042/202326
Project section - Investigating Aromaticity
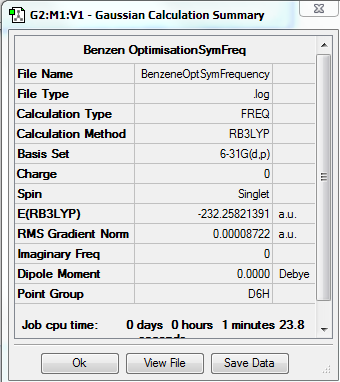
Benzene
Method - RB3LYP
Basis set - 6-31G(d.p)
Item Value Threshold Converged? Maximum Force 0.000198 0.000450 YES RMS Force 0.000087 0.000300 YES Maximum Displacement 0.000757 0.001800 YES RMS Displacement 0.000321 0.001200 YES Predicted change in Energy=-3.962814D-07 Optimization completed.
Low frequencies --- -2.1456 -2.1456 -0.0088 -0.0042 -0.0042 10.4835 Low frequencies --- 413.9768 413.9768 621.1390
test molecule |
File:BenzeneOptSymFrequency.log
Charge Distribution of Benzene
Charge for Carbon atoms (Red) = -0.239
Charge for Hydrogen atoms (Green) = 0.239
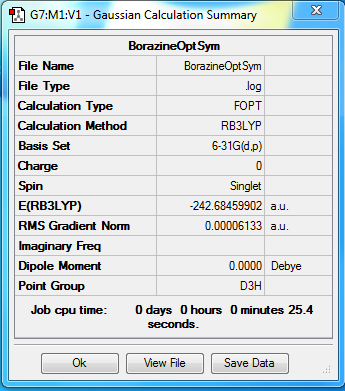
Borazine
Method - RB3LYP
Basis set - 6-31G(d.p)
Item Value Threshold Converged? Maximum Force 0.000211 0.000450 YES RMS Force 0.000067 0.000300 YES Maximum Displacement 0.000317 0.001800 YES RMS Displacement 0.000102 0.001200 YES Predicted change in Energy=-1.167390D-07 Optimization completed.
Low frequencies --- -12.8174 -12.4924 -8.9689 -0.0110 0.0406 0.0628 Low frequencies --- 289.0994 289.1108 403.8686
test molecule |
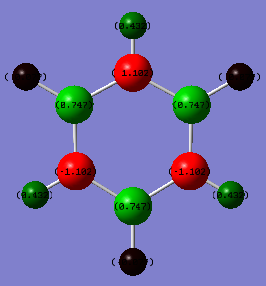
Charge Distribution of Borazine
Charge for Nitrogen atoms (Red) = -1.102
Charge for Boron atoms (Light Green) = 0.747
Charge for Hydrogen atoms connected to Nitrogen (Dark Green) = 0.432
Charge for Hydrogen atoms connected to Boron (Black) = 0.077
Comparison between Benzene and Borazine
Charge Comparison
| Molecule | Charge(NBO) | Charge (Colour) |
|---|---|---|
| Benzene | 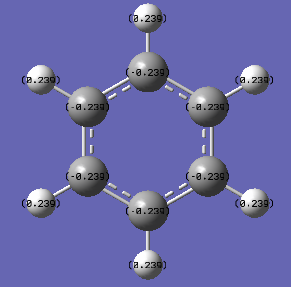 |
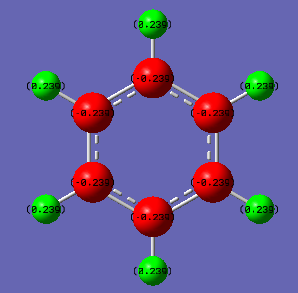
|
| Borazine | 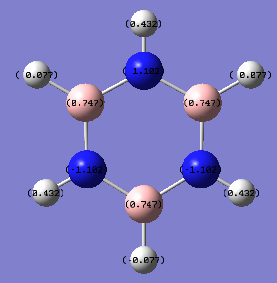 |
|
The difference between the charges in the two ring is essentially down to the electronegativity difference between the atoms. In Benzene the ring is comprised of purely Carbons, so the electronegativity difference is zero. Where as in Borazine the ring is comprised of Nitrogens and Borons. The electronegativity of Nitrogen is 3.04 and the electronegativity of Boron is 2.04, giving an electronegativity difference of 1. This leads to Borazines ability to experience both electrophilic and nucleophilic attack, due to the fact that the atoms within the ring have different charges. In contrast, Benzene can only experience electrophilic attack.
MO Comparison
Smf115 (talk) 07:20, 17 May 2018 (BST)Very good comparison of the MOs with correct terminology to desribe them. A nice range of MOs were chosen and it's good to see MO 24 used!
The Concept of Aromaticity
The concept of aromaticity revolves around Hückels rule. Hückels rule states that: -the molecule must have 4n+2 electrons in a conjugated system of p orbitals. -the molecule must approximately planar so that the p orbitals are roughly parallel and so can interact. -the molecule must be closed and cyclic. -the molecule must have a continuous ring of p orbitals (in essence, no sp3 atoms on the ring). If a molecule obeys all these rules but the first and instead contains 4n molecules then it is known as anti-aromatic.
The structure of the aromatic molecule benzene was a topic of confusing initially, at first it was thought to be a cyclic molecule with alternating double bonds. However though looking at the bond lengths this theory was disproved. The bond length of a single C-C bond is 1.54 Angstroms and the bond length of a double C=C bond is 1.34 Angstroms, however the bond length seen was 1.40 Angstroms, an intermediate bond length. The eventual theory that has been accepted is that the electron density is spread evenly around the whole ring through a π bond above and below the ring, delocalising the orbitals and sharing the atoms. So rather than forming double bonds, the extra electron density strengthens all of the bonds evenly, making them stronger than a C-C bond but not as strong as a C=C bond. Aromatic molecules exhibit additional stability to what is expected due to the conjugation of the molecules, making them hard to break up/react with.
The MOs generated through Gaussview have varying degrees of accuracy when compared to those drawn through the LCAO method. This is because the LCAO diagram is very accurate at low levels of complexity, however begins to fall off with more difficult molecules. For molecules such as Benzene which are more complex but has a high level of symmetry then the LCAO does a decent job at expressing it accurately. However with aromatic molecules with lower levels of symmetry the LCAO method struggles due to the fact it struggles to cope with heteroatomic rings and their differences in electronegativity.
The concept of overlapping pz AOs is not a good description for aromaticity as with heteroatomic rings the pz orbitals cant overlap as well, even though the molecule is considered aromatic and so the electrons cannot delocalise as well.
Smf115 (talk) 07:20, 17 May 2018 (BST)The key concepts of aromaticity are well discussed. Further consideration of the MOs, with reference to those just viewed in benzene and borazine, and how they can illustrate how the usual pZ AO overlap isn't a good descirptor of aromaticity would be a way to improve. Further development into the more complex descriptions of aromaticity would also be good to see.
Smf115 (talk) 07:24, 17 May 2018 (BST)Overall a good wiki report with some further ideas required within the aromaticity and charge analysis discussion. Well presented and a good MO comparison in the project section.