Rep:Mod:Andrew Kikomeko InorganicComupational
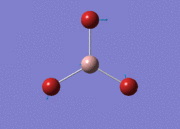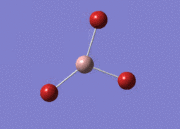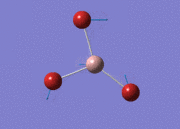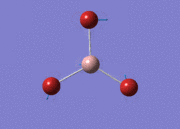
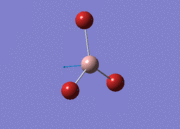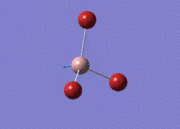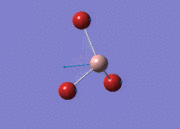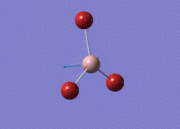
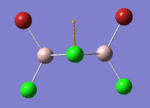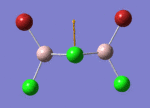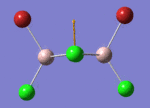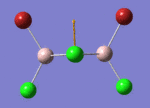
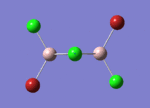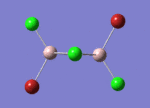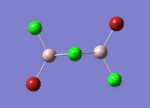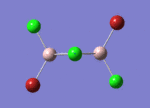
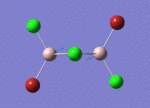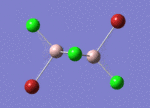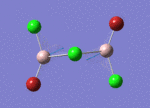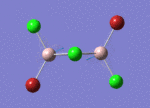
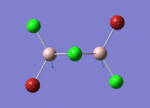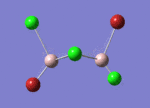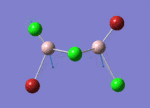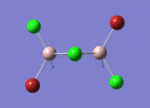
BH3
Optimisation of BH3,Method B3LYP, basic set 3-21G
Log file : bh3_opt.LOG
Gaussian calculation summary for BH3 optimistion : GCS_BH3_OPT
optimised B-H bond distance = 1.19349 Å optimised H-B-H bond angle = 120.0
Item Value Threshold Converged?
Maximum Force 0.000413 0.000450 YES
RMS Force 0.000271 0.000300 YES
Maximum Displacement 0.001610 0.001800 YES
RMS Displacement 0.001054 0.001200 YES
Predicted change in Energy=-1.071764D-06
Optimization completed.
-- Stationary point found.
Optimisation complete as forces have all converged
Optimisation of BH3,Method B3LYP, basic set 6-31G(d,p) Log file : bh3_opt_6-31G(d,p)
Gaussian calculation summary for BH3 optimistion with basic set 6-31G(d,p), : GCS_BG3_OPT_631GDP
Item Value Threshold Converged?
Maximum Force 0.000005 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000019 0.001800 YES
RMS Displacement 0.000012 0.001200 YES
Predicted change in Energy=-1.305135D-10
Optimization completed.
-- Stationary point found.
Optimisation complete as forces have all converged
optimised B-H bond distance = 1.19232 Å optimised H-B-H bond angle = 120.0
Optimised BH3 toatal energies
Total energy for 3-21G optimised BH3 = -26.46226338 a.u.
Total energy for 6-31G(d,p) optimised BH3 = -26.61532363 a.u.
total energy difference = 0.15306025 a.u. = 401.85971699 Kj/mol
The difference in energy shows that using a higher accuracy basic set 6-31G(d,p) produce a structure with a lower energy than that with the lower accuracy basis set of 3-21G.
GaBr3
Optimisation of GaBr3, method B3LYP , basis set LANL2DZ
Log file :GaBr3_OPT.LOG
Gaussian calculation summary : summary_GaBr3_opt.log
Link to D-space : [1]
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000003 0.001800 YES
RMS Displacement 0.000002 0.001200 YES
Predicted change in Energy=-1.282683D-12
Optimization completed.
-- Stationary point found.
Optimisation complete as forces have all converged
optimised Ga-Br bond distance = 2.35234 Å optimised Br-Ga-Br bond angle = 120
Literature comparison
Literature Ga-Br bond distance = 2.35 Å[1]
Calculated Ga-Br bond distance = 2.35 Å
Using the pseudo potentials has optimised my molecule gave the same bond length to the literature value.This means that the calculation used on Gauss-view give am extremely good estimate of the actual bond information .
BBr3
optimisation, Method = B3LYP, Basis set = Gen
Log file : BBr3_opt
Gausssian calculation Summary : summary_BBr3
BBr3 optimisation D-space link, [2]
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000036 0.001800 YES
RMS Displacement 0.000023 0.001200 YES
Predicted change in Energy=-4.027283D-10
Optimization completed.
-- Stationary point found.
Optimisation complete as forces have all converged
Structure comparison
| Molecule | Bond length (Å) |
|---|---|
| BH3 | 1.19232 |
| BBr3 | 1.93396 |
| GaBr3 | 2.35234 |
The bond lengths follow the order B-H < B-Br < Ga-Br .On changing the ligand as with BH3 to BBr3 the bond length increases, this could be because of sterics the H atoms are much smaller the the Br atoms so they can get closer to the central B atom, also Br has larger valance orbitals so there will be a poorer overlap with the B orbitals so they form longer bonds. on changing the central atom as with BBr 3 to GaBr3 the bond length increased, this is because Ga is lower down in the periodic table so will have larger more diffuse orbitals meaning a poorer overlap with the Br orbitals gives a larger bond.Ga and B produce similar structures as they are both high up in group 13 so have the same same number of valance electrons. Throughout all the structures the optimised bond angles were all 120, this was expected as all of the molecules were planar.
In gaussview the bonds that we originally drew were just to help us visualise the structure of the molecule so gaussview takes these bond distance, once the structure was optimised the bond lenghts would have changed from those first drawn and gaussview does not recognise the new optimised bond even thought there is a bond but not shown.
BH3 frequency analysis
Calculation Type = FREQ, Calculation Method = RB3LYP, Basis Set = 6-31G(d,p)
Log file :Freq_BH3.LOG
Gaussian calculation summary : BH3_freq_sum.log
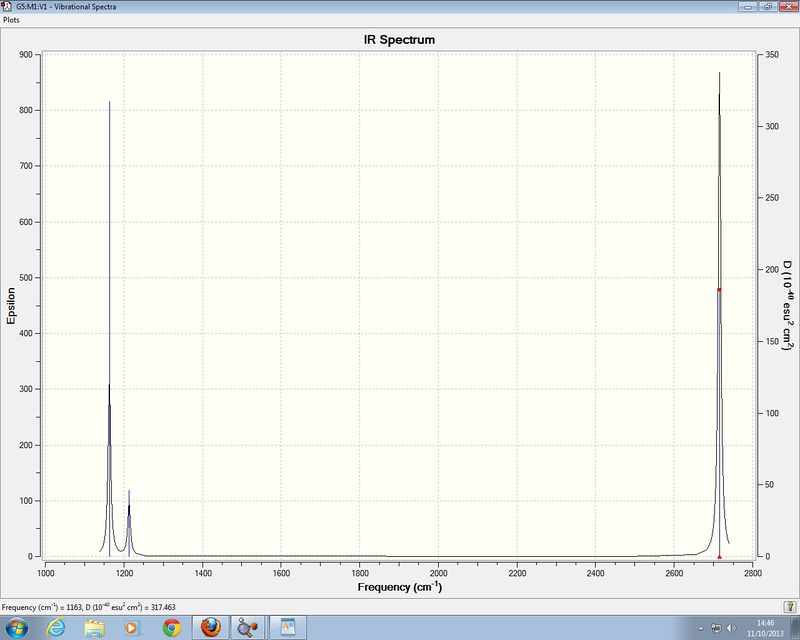
Low frequencies --- -0.9033 -0.7343 -0.0054 6.7375 12.2491 12.2824 Low frequencies --- 1163.0003 1213.1853 1213.1880
There are six vibrational modes present for BH3 but only 3 peaks can be seen on the IR spectrum. Firstly for a vibrational mode to be IR active there must be a change in the dipole moment of the molecule for IR radiation to be absorbed. For the vibrational mode with frequency 2582 cm-1 the intensity is zero which means that as the vibration is symmetric there will be no change in the dipole moment of the vibration so it does not appear in the IR spectrum. There are also two sets of vibrations have the same symmetry label and so absorb at the same frequency and have the same intensity so these peaks have merged to form only two peaks.
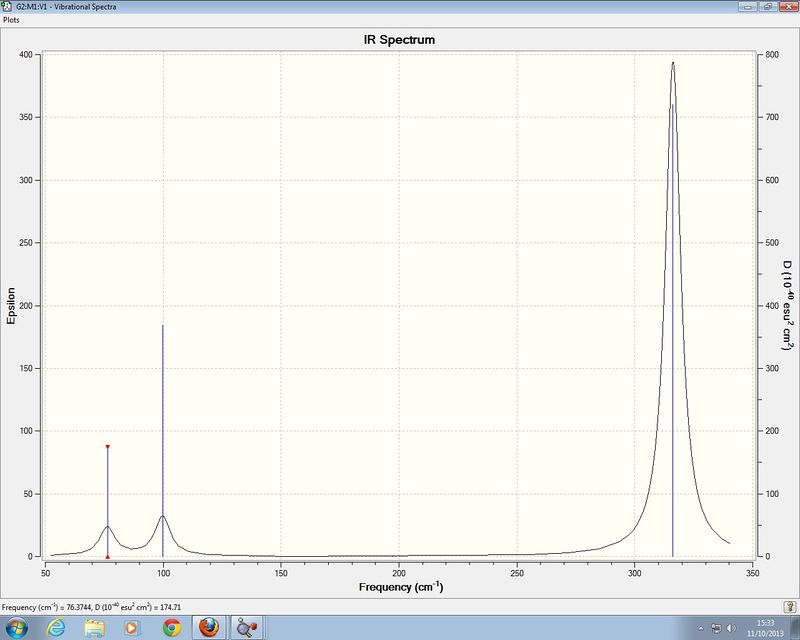
GaBr3 Frequency analysis
Log file : GaBr3_frequency_analysis.LOG
Gausssian calculation summary : summary_GaBr3_freq
Frequency analysis D-space link : [3]
Low frequencies --- -0.5252 -0.5247 -0.0024 -0.0010 0.0235 1.2010 Low frequencies --- 76.3744 76.3753 99.6982
IR spectrum of GaBr3
Comparing the vibrational frequencies of BH 3and GaBr 3
The large difference in the values of the frequencies between BH3 and GaBr3 indicate that the bonds formed in Bh3 are strong than those formed in Gabr3. Since Ga and Br are both larger atoms than those in BH3 there bonds will require less energy to vibrate as heavier atom vibrate at lower frequencies. There has been a recording of modes in each spectra As both molecules have the same point group they will have vibrations of similar symmetry as seen by both molecules have vibrational modes of A1', A2and two sets of E' vibrational modes A1' and E' modes lie higher in energy because the vibrations associated with these stretches involve strechting of bonds whilst the A2 and E' modes only involve bending and scissoring of the bonds which requires less energy than a bond stretch.
The same method and basis set for both the optimisation and frequency analysis calculations because we want to ensure that the same claculations are used that optimised the geomerty of the molecule under study. The purpose of carrying out a frequency analysis is to confirm we have minimum energy of the structures we have optimised. Molecules with N atoms will have 3N-6 normal vibrational frequencies. The "Low frequencies" represent -6 and are the motions of the centre of mass of the molecule.
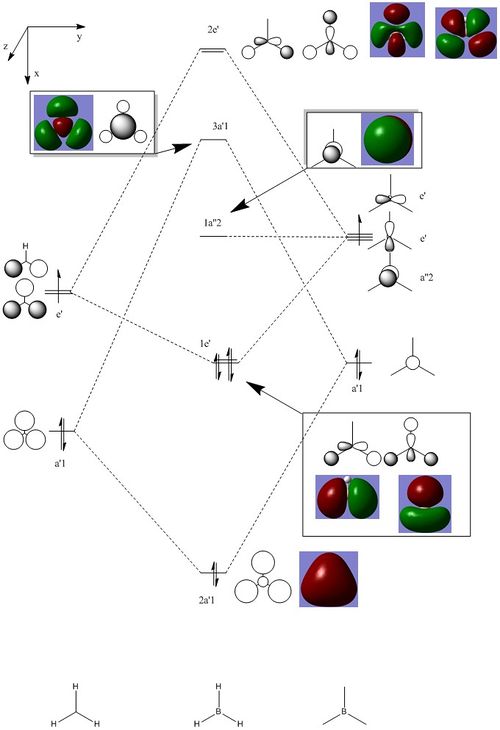
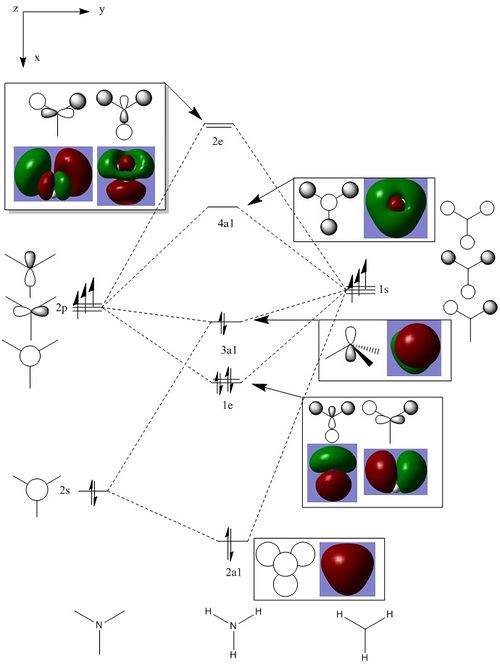
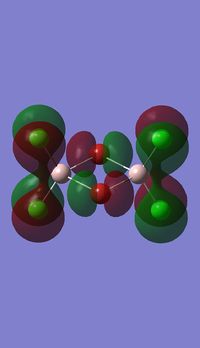
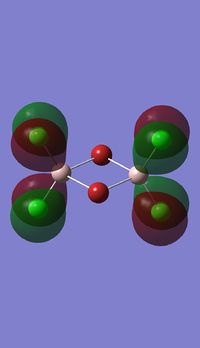
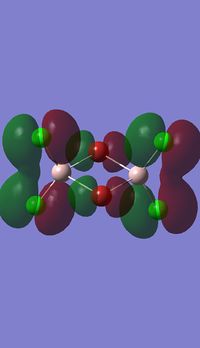
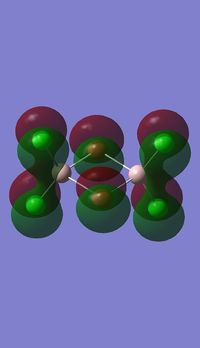
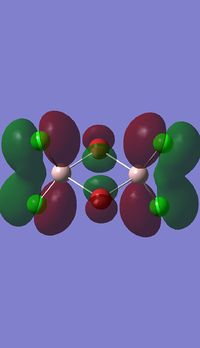
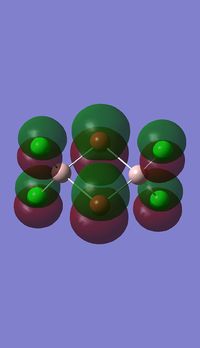
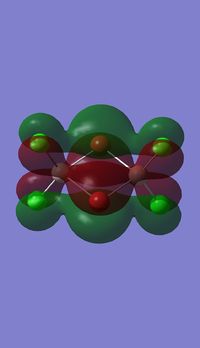
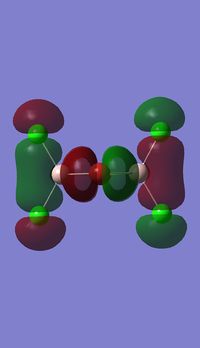
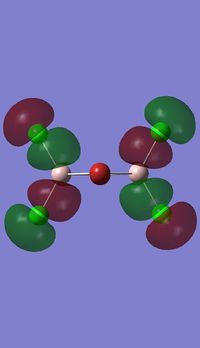
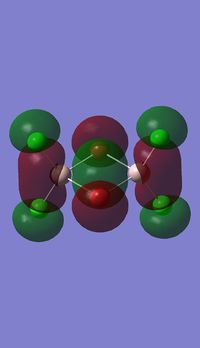
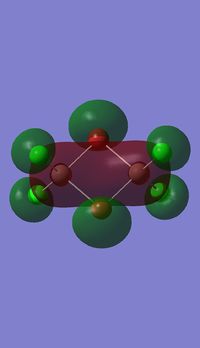
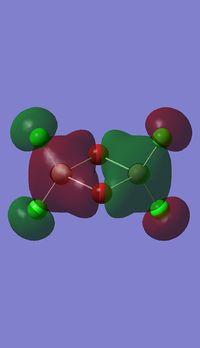
Molecular orbitals of BH3
The checkpoint file of the higher basis set optimised BH3 molecule was opened, the job type was changed to Energy,FULL NBO was turned on and the keyword pop=full entered and then the job was submitted to gaussian.
log file : MO_BH3.LOG
Gaussian calculation summary : summary.log
Molecule orbital diagram of BH3
Molecule orbital diagram built in ChemDraw Pro 13.0
There appears to be no significant differences between the real and LCAO MO, the LCAO MOs produce a good initial estimate of what the real MOs made look like. The accuracy and usefulness of qualitative MO theory is good.
NBO Analysis
Optimisation of NH3
Higher level basis set6-31G(d,p) ,Method B3LYP, keyword nosymm
Log file :NH3 optimisation.LOG
Guassian calculation Summary : NH3 opt summary
Item Value Threshold Converged?
Maximum Force 0.000005 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000010 0.001800 YES
RMS Displacement 0.000007 0.001200 YES
Predicted change in Energy=-7.830245D-11
Optimization completed.
-- Stationary point found.
optimised N-H bond distance = 1.01797 angstroms , optimised H-N-H bond angle = 105.746 , Total energy of optimised NH3 = -56.55776863 a.u.
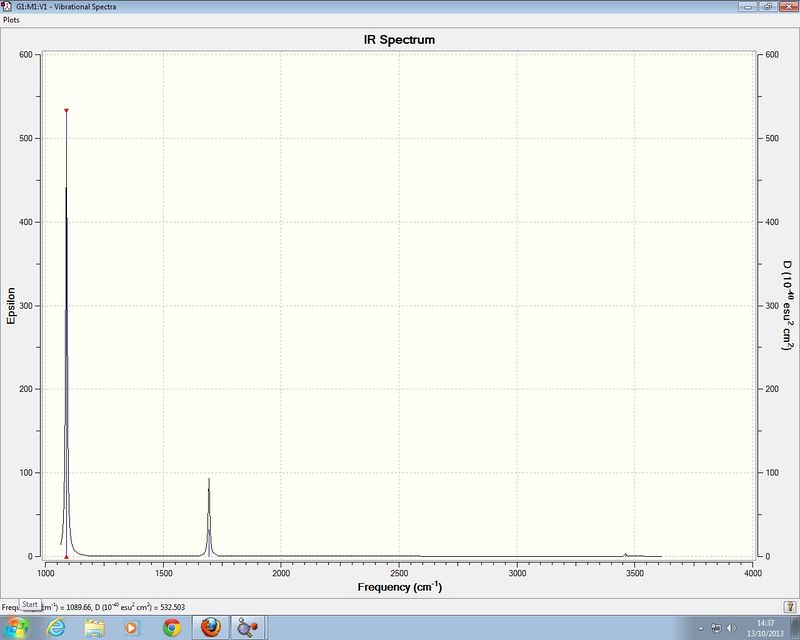
Frequency analysis of NH3
Log file :NH3_Freq.LOG
Gaussian Frequency calculation Summary : NH3_Freq_Summary.LOG
Low frequencies --- -11.6223 -11.5869 -0.0029 0.0244 0.1403 25.5604 Low frequencies --- 1089.6629 1694.1734 1694.1737
| No. | Form of frequency | Frequency | Intensity | Symmetry of point group | |
|---|---|---|---|---|---|
| 1 |  |
1089.66 | 145 | A1 | |
| 2 | 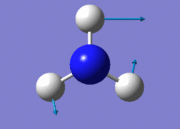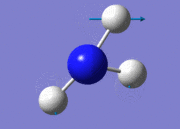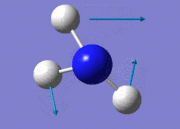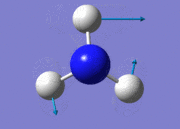 |
1694.17 | 14 | E | |
| 3 | 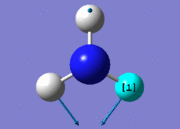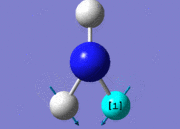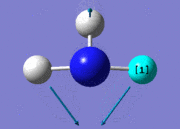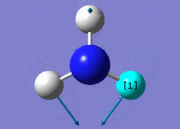 |
1694.17 | 14 | E | |
| 4 |  |
3461.07 | 1 | A1 | |
| 5 |  |
3589.55 | 0 | E | |
| 6 |  |
3589.55 | 0 | E |
IR spectrum of NH3
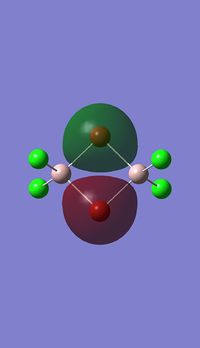
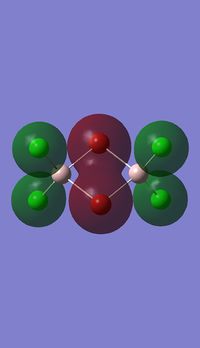
Molecualr orbitals of NH3
Log file :MO_NH3.LOG
Gausssian calculation summary : SUMMARY_MO_NBOFULL.LOG NH3 MO diagram
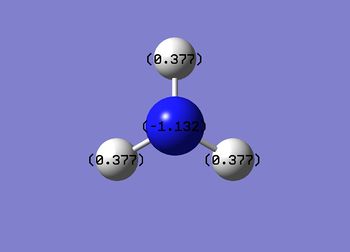
Charge Distribution
 Charge range = -1.132 to 1.132
Charge range = -1.132 to 1.132
Specific NBO charges
N = -1.132 ,H = 0.377
Association energy NH3BH 3
NH3BH 3 Optimisation
My molecule of NH3BH 3 was optimised using the higher basis set of 6-31G(d,p) with method b3lyp.
Log file : NH3BH3_OPT.LOG
Gaussian calculation Summary : NH3BH3_SUMMARY.LOG
Item Value Threshold Converged? Maximum Force 0.000139 0.000450 YES RMS Force 0.000063 0.000300 YES Maximum Displacement 0.000771 0.001800 YES RMS Displacement 0.000338 0.001200 YES
The forces have successfully converged
NH3BH 3 Frequency analysis
Log file : NH3BH3_Freq.LOG
Gaussian calculation Summary : Summary_NH3BH3.LOG
Low frequencies --- -799.6042 -403.2532 -403.2449 -0.0047 -0.0029 0.0073 Low frequencies --- 640.8431 640.8490 911.9308
Energies
E(NH3) = -56.55776863 a.u.
E(BH3) = -26.61532363 a.u.
E(NH3BH3) =-83.18367495 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)] = -0.01058269 a.u.
Association energy ( Kj/mol) = -27.7848547115 kj/mol
The dissociation energy is exothermic,(27.7848547115kj/mol) so forming the bond between N and the B releases energy as the dative bond from the nitrogen lone pair is formed.
Lewis acids and bases
Introduction
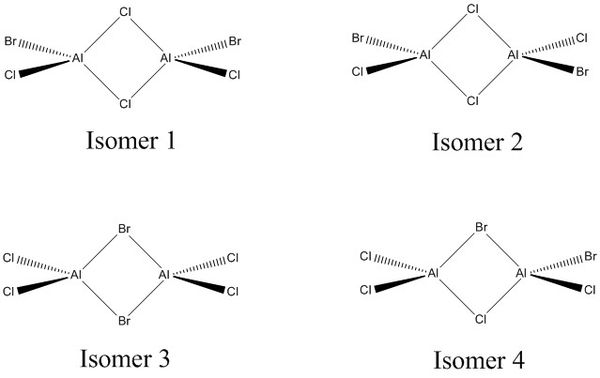
The project studies the electron deficient AlCl2Br molecule and the conformers that can be formed from the dimer 2AlCl2Br. The four possible isomers that can be formed from two AlCl 2 Br monomers are;
I will optimise the structures and and then compare and study the conformers using frequency then MO analysis
AlCl2Br Isomer 1
AlCl2Br Isomer 1 Optimisation was initially carried out using the lower level basis set 3-21G so that i could obtain a good basic approximation of the structure, then after this was completed a mixed psueod-potential LanL2DZ was used on the heavier Br atoms and a higher basis sets 6-31G(d,p) was used the smaller Al and Cl atoms.
Log file : AlCl2Br_Opt_321G.LOG
Gausssian calcualtion summary : Summary_AlClBr_OPT_321G.LOG
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25593
Item Value Threshold Converged?
Maximum Force 0.000095 0.000450 YES
RMS Force 0.000021 0.000300 YES
Maximum Displacement 0.001016 0.001800 YES
RMS Displacement 0.000358 0.001200 YES
Predicted change in Energy=-6.174960D-08
Optimization completed.
-- Stationary point found.
AlCl2Br Optimisation :Method B3LYP, basic set 6-31G(d,p) for Al and Cl and PP LANL2DZdp on Br
Log file : AlCl2Br_OPT_GEN.LOG
Gausssian calculation summar : Summary_AlCl2Br_OPT_GEN.LOG
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25600
Item Value Threshold Converged?
Maximum Force 0.000039 0.000450 YES
RMS Force 0.000016 0.000300 YES
Maximum Displacement 0.001350 0.001800 YES
RMS Displacement 0.000419 0.001200 YES
Predicted change in Energy=-2.512449D-08
Optimization completed.
-- Stationary point found.
| Bond | Bond length (A) |
|---|---|
| Br-Al | 2.27465 |
| tCl-Al | 2.09389 |
| μCl-Al | 2.29816 |
| Bond | Bond angle |
|---|---|
| μCl-Al-μCl | 90.164 |
| Al-μCl-Al | 89.834 |
| Br-Al-μCl | 110.510 |
| Br-Al-tCl | 121.504 |
| tCl-Al-μCl | 109.851 |
2AlCl 2 Br Isomer 1 Frequency analysis of 2nd Optimistion
Isomer 1 of 2AlCl 2 Br has C2v symmetry , for the frequency analysis C2v symmetry was imposed on the isomer. I was able to confirm that a minimum .
Log file : AlCl2Br_Freq.LOG
Gaussian calculation Summary : Summary_AlCl2Br_Freq.LOG
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25636
Low frequencies --- -3.8098 -2.2404 0.0021 0.0030 0.0041 1.4034 Low frequencies --- 17.2042 50.9468 78.5401
Isomer 1 AlCl 2 Br has C2v symmetry
| Mode | Frequency | Intensity | Symmetry of C2v point group |
|---|---|---|---|
| 1 | 17 | 0 | A1 |
| 2 | 51 | 0 | A2 |
| 3 | 79 | 0 | A1 |
| 4 | 99 | 0 | B2 |
| 5 | 103 | 3 | B1 |
| 6 | 121 | 13 | B2 |
| 7 | 123 | 6 | B1 |
| 8 | 157 | 0 | A2 |
| 9 | 159 | 5 | A1 |
| 10 | 194 | 2 | A1 |
| 11 | 264 | 0 | A2 |
| 12 | 279 | 25 | B2 |
| 13 | 309 | 2 | A1 |
| 14 | 413 | 149 | B1 |
| 15 | 420 | 411 | B2 |
| 16 | 461 | 35 | A1 |
| 17 | 570 | 32 | B2 |
| 18 | 582 | 278 | A1 |
AlCl2Br Isomer 2
AlCl2Br Isomer 2 Optimisation was initially carried out using the lower level basis set 3-21G so that i could obtain a good basic approximation of the structure, then after this was completed a mixed psueod-potential LanL2DZ was used on the heavier Br atoms and a higher basis sets 6-31G(d,p) was used the smaller Al and Cl atoms.
Lower level basis set 3-21G optimisation
Log file:AlCl2Br_Isomer2_Opt_3-21G.LOG
Gaussian calcualtion summary file : AlCl2Br_isomer2_opt_3-21G_summary.LOG
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25599
Item Value Threshold Converged?
Maximum Force 0.000015 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000938 0.001800 YES
RMS Displacement 0.000276 0.001200 YES
Predicted change in Energy=-5.687069D-09
Optimization completed.
-- Stationary point found.
forces have converged
Mixture of higher basis sets 6-31G(d,p) and psueod-potentials LanL2DZ
Log file : AlCl2Br_Isomer2_opt_Gen.LOG
Gaussian calculation summary file : AlCl2Br_Isomer2_opt_Gen_Summary.LOG
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25619
Item Value Threshold Converged?
Maximum Force 0.000039 0.000450 YES
RMS Force 0.000015 0.000300 YES
Maximum Displacement 0.000466 0.001800 YES
RMS Displacement 0.000168 0.001200 YES
Predicted change in Energy=-2.438748D-08
Optimization completed.
-- Stationary point found.
Forces have converged, so now i can continue and carry out a frequency analysis.
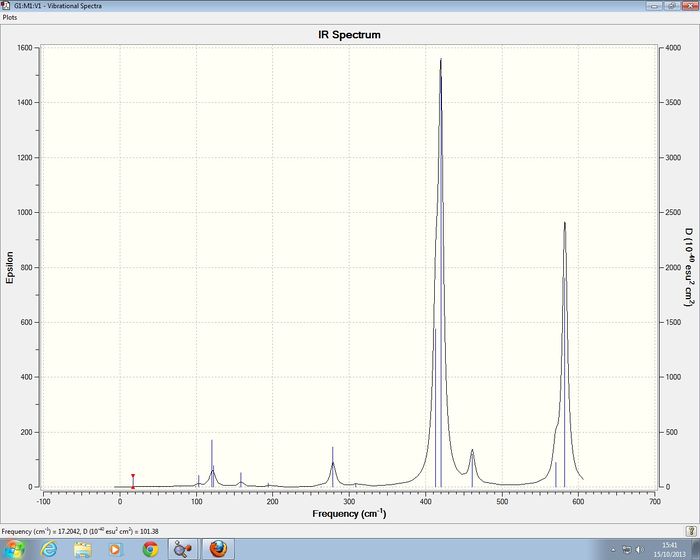
2AlCl 2 Br Isomer 2 Frequency analysis of 2nd Optimistion
Isomer 2 of 2AlCl 2 Br has C2h symmetry , for the frequency analysis C2h symmetry was imposed on the isomer. I was unable to confirm that a minimum had been reach as i had no imposed the symmerty constant during the optimisation so a slightly different energy was obtained.
Log file : AlCl2Br_Isomer2_Freq.LOG
Gaussian calculation summary : Summary_AlCl2Br_Isomer2_Freq.LOG
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25639
Full mass-weighted force constant matrix: Low frequencies --- 0.0029 0.0034 0.0039 1.8476 1.9593 4.1058 Low frequencies --- 18.1144 49.0897 72.9230
No negative frequencies were obtained , the largest zero frequency obtained was 4.1058 which is within reason close to 0.
| Mode | Frequency | Intensity | Symmetry of C2h point group |
|---|---|---|---|
| 1 | 18 | 0 | Bu |
| 2 | 49 | 0 | Au |
| 3 | 73 | 0 | Ag |
| 4 | 105 | 0 | Ag |
| 5 | 110 | 0 | Bg |
| 6 | 117 | 9 | Au |
| 7 | 120 | 13 | Bu |
| 8 | 157 | 0 | Bg |
| 9 | 160 | 3 | Bu |
| 10 | 192 | 0 | Ag |
| 11 | 264 | 0 | Bg |
| 12 | 280 | 29 | Bu |
| 13 | 308 | 0 | Ag |
| 14 | 413 | 149 | Au |
| 15 | 421 | 438 | Bu |
| 16 | 459 | 0 | Ag |
| 17 | 574 | 0 | Ag |
| 18 | 579 | 316 | Bu |
2 AlCl 2 Br Isomer 3
2AlCl2Br Isomer 3 Optimisation was initially carried out using the lower level basis set 3-21G so that i could obtain a good basic approximation of the structure, then after this was completed a mixed psueod-potential LanL2DZ was used on the heavier Br atoms and a higher basis sets 6-31G(d,p) was used the smaller Al and Cl atoms. AlCl2Br Isomer3 Optimisation :Method B3LYP
Lower level basis set 3-21G optimisation
Log file : AlCl2Br_Isomer3_Opt_3-21G.LOG
Gaussian calculation summary : AlCl2Br_isomer3_opt_3-21G_summary.LOG
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25603
Item Value Threshold Converged?
Maximum Force 0.000021 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000536 0.001800 YES
RMS Displacement 0.000164 0.001200 YES
Predicted change in Energy=-6.461905D-09
Optimization completed.
-- Stationary point found.
Mixture of higher basis sets 6-31G(d,p) and psueod-potentials LanL2DZ optimisation
Log file : AlCl2Br_Isomer3_opt_Gen.LOG
Gaussian calculation summary :AlCl2Br_Isomer3_opt_Gen_Summary.LOG
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25620
Item Value Threshold Converged?
Maximum Force 0.000007 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000182 0.001800 YES
RMS Displacement 0.000060 0.001200 YES
Predicted change in Energy=-1.218594D-09
Optimization completed.
-- Stationary point found.
forces have converged
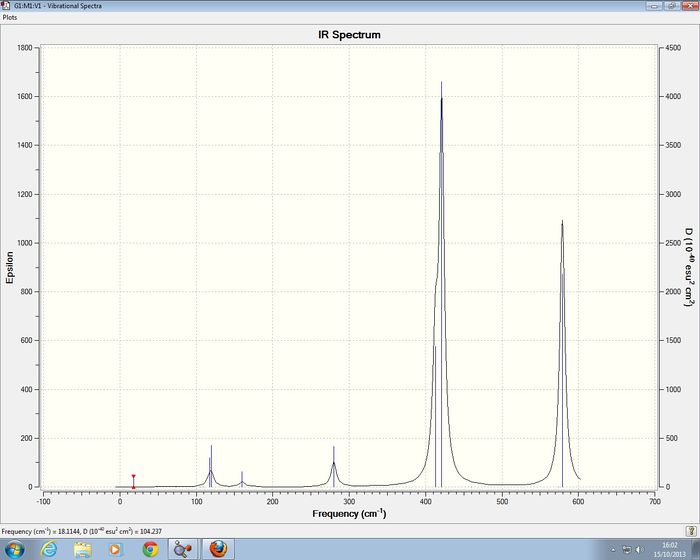
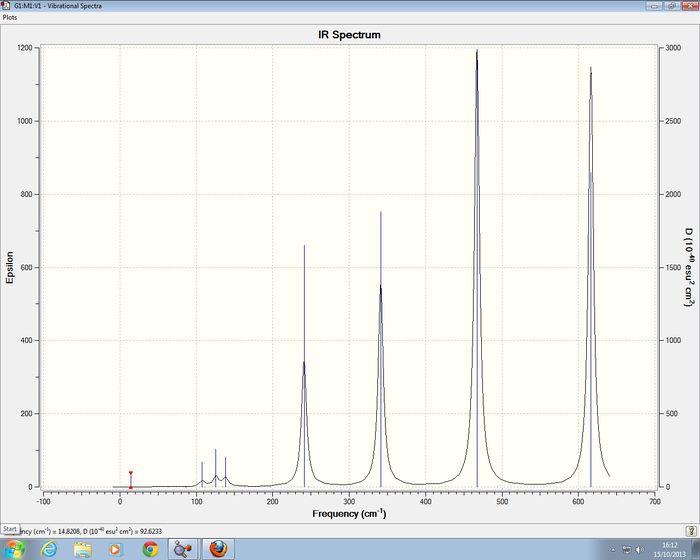
2AlCl 2 Br Isomer 3 Frequency analysis of 2nd Optimistion conditions
Isomer 3 of 2AlCl 2 Br has D2h symmetry , for the frequency analysis D2h symmetry was imposed on the isomer. I was able to confirm that a minimum had been reach.
Log file : AlCl2Br_Isomer3_Freq.LOG
Gaussian calculation Summary : Summary_AlCl2Br_Isomer3_Freq.LOG
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25640
Low frequencies --- -5.1945 -5.0514 -3.2258 -0.0036 -0.0035 -0.0030 Low frequencies --- 14.8208 63.2774 86.0814
Isomer 3 of 2AlCl 2 Br has D2h symmetry
| Mode | Frequency | Intensity | Symmetry of D2h point group |
|---|---|---|---|
| 1 | 15 | 0 | B2u |
| 2 | 63 | 0 | Au |
| 3 | 86 | 0 | B3g |
| 4 | 87 | 0 | Ag |
| 5 | 108 | 5 | B1u |
| 6 | 111 | 0 | B1g |
| 7 | 126 | 8 | B3u |
| 8 | 135 | 0 | B2g |
| 9 | 138 | 7 | B2u |
| 10 | 163 | 0 | Ag |
| 11 | 197 | 0 | E2g |
| 12 | 241 | 100 | B3u |
| 13 | 247 | 0 | Ag |
| 14 | 341 | 161 | B1u |
| 15 | 467 | 347 | B3u |
| 16 | 494 | 0 | Ag |
| 17 | 608 | 0 | B1g |
| 18 | 616 | 332 | B2u |
2 AlCl 2 Br Isomer 4
2AlCl2Br Isomer 4 Optimisation was initially carried out using the lower level basis set 3-21G so that i could obtain a good basic approximation of the structure, then after this was completed a mixed psueod-potential LanL2DZ was used on the heavier Br atoms and a higher basis sets 6-31G(d,p) was used the smaller Al and Cl atoms. 2AlCl2Br Isomer4 Optimisation :Method B3LYP A
Lower level basis set 3-21G optimisation
Log file : AlCl2Br_Isomer4_Opt_3-21G.LOG
Gaussian calculation summary : AlCl2Br_isomer4_opt_3-21G_summary.LOG
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25605
Item Value Threshold Converged?
Maximum Force 0.000017 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.001071 0.001800 YES
RMS Displacement 0.000386 0.001200 YES
Predicted change in Energy=-1.178876D-08
Optimization completed.
-- Stationary point found.
forces have converged
Mixture of higher basis sets 6-31G(d,p) and psueod-potentials LanL2DZ optimisation
Log file : AlCl2Br_Isomer4_opt_Gen.LOG
Gaussian calculation summary : AlCl2Br_Isomer4_opt_Gen_Summary.LOG
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25617
Item Value Threshold Converged?
Maximum Force 0.000036 0.000450 YES
RMS Force 0.000014 0.000300 YES
Maximum Displacement 0.000635 0.001800 YES
RMS Displacement 0.000198 0.001200 YES
Predicted change in Energy=-2.219690D-08
Optimization completed.
-- Stationary point found.
forces have converged
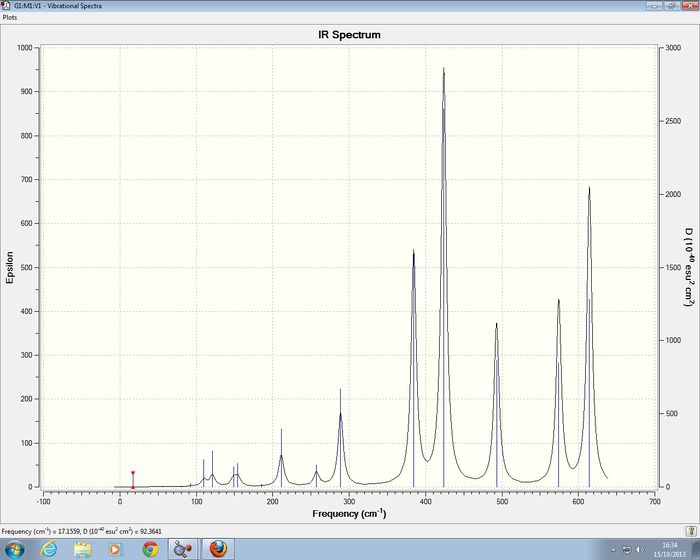
2AlCl 2 Br Isomer 4 Frequency analysis of 2nd Optimistion conditions
Isomer 4 of 2AlCl 2 Br has C1 symmetry , for the frequency analysis C1 symmetry was imposed on the isomer. I was able to confirm that a minimum had been reach.
Log file : AlCl2Br_Isomer4_Freq.LOG
Gauassian calculation Summary : Summary_AlCl2Br_Isomer4_Freq.LOG
Link to D-Space:https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25641
Low frequencies --- -2.2844 -0.0022 -0.0016 0.0015 1.3995 3.2655 Low frequencies --- 17.1598 55.9534 80.0568
Isomer 4 of 2AlCl 2 Br has C1 symmetry
| Mode | Frequency | Intensity | Symmetry of C1 point group |
|---|---|---|---|
| 1 | 17 | 0 | A |
| 2 | 56 | 0 | A |
| 3 | 80 | 0 | A |
| 4 | 92 | 1 | A |
| 5 | 107 | 0 | A |
| 6 | 110 | 5 | A |
| 7 | 121 | 8 | A |
| 8 | 149 | 5 | A |
| 9 | 154 | 6 | A |
| 10 | 186 | 1 | A |
| 11 | 211 | 21 | A |
| 12 | 257 | 10 | A |
| 13 | 289 | 48 | A |
| 14 | 384 | 153 | A |
| 15 | 424 | 274 | A |
| 16 | 493 | 107 | A |
| 17 | 574 | 122 | A |
| 18 | 61 | 197 | A |
IR Analysis
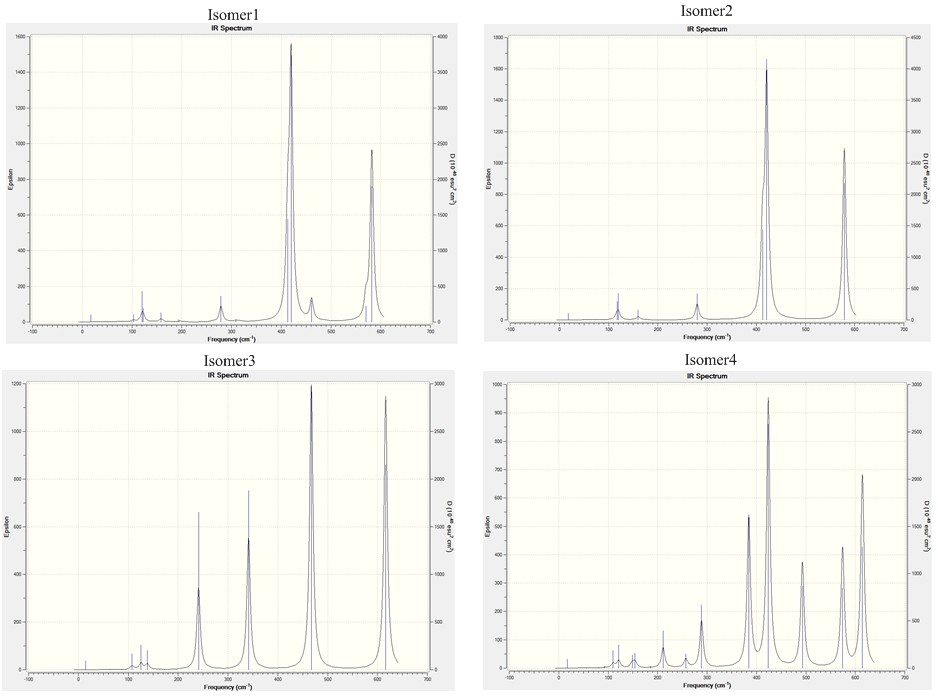
Firstly the requirement for an IR band to be active is the there must be a change in the dipole moment during the vibration for infrared energy to be adsorbed. This means that highly symmetric molecules should have fewer observable vibrational modes in there IR spectra.
The IR spectra of the four comformers show a varying number of observable bands, this is due to the higher degrees symmetry of seen amongst the four isomers. The least symmetric conformer is isomer 4 with point group C1, its IR spectra has the most bands which is . The most symmetric isomers are isomer 2 and isomer 3, they have the least number of observable bands present in their IR spectra seen by the large number of zero intensity vibration modes.
Al-Br stretching vibrations with respect to the terminal and bridging position of the Br atom
I have tabulated the Vibrational modes which show Al-Br stretching, for isomer 1 and isomer 2 the Br atoms are in the terminal positions (tBr-Al) and for isomers 3 Br atoms are both bridging μμBr-Al ,and finally in isomer 4 there is terminal and briging Br atoms.
For the 1st an 2nd isomers the key Al-Br stretching vibrations appear to be the modes; 13,15,16,17,and 18.The first key differece is that for isomer 2 there are two vibrational modes (16 and 17) which have an intensity of 0 as these vibrations lead to no changes in the diople moment of the molecule. The highest frequency vibrations for both isomers are the Mode 18 vibrations of 582 cm-1 and 579cm-1 for isomer 1 and isomer 2 respectively, which have intensities of 278 and 316.The lowest frequency vibrational mode is the mode 13 of of 309 cm-1 and 421-1 for isomer 1 and isomer 2 respectively, which have intensities of 2 and 438.4061. For the vibration mode 13 for isomers 1 and 2, there is a large differnece in the frequency of the vibration, this is due to the In both cases here the intensity of the isomer 2 tBr-Al stretches is larger than those of isomer 1, this is due to the lower symmetry of isomer 2 which allows the vibrations to cause a greater change in the dipole.'
On comparing the vibrational mode 13 for all of the isomers it can be seen that isomers 1 and isomer 2have a similar frequency, however isomers 3 and isomer 4 which contain bridging Br atoms have a smaller vibrational frequency. This lowered vibrational frequency can be explained in terms of the weaker bond formed to the bridging Br atom so it requires less energy to vibrate, compared to the terminal bonds which are stronger so have the higher frequency of vibration.
Analysing the energy of the 4 conformers
| Conformer | Energy (a.u.) | Energy (Kj/mol) | Energy relative compared to lowest energy conformer (Kj/mol) |
|---|---|---|---|
| Isomer 1 | -2352.41626677 | -6176269.37888789 | 26.14680514 |
| Isomer 2 | -2352.41631609 | -6176269.50837756 | 26.27629481 |
| Isomer 3 | -2352.40630798 | -6176243.23208275 | 0 |
| Isomer 4 | -2352.41109943 | -6176255.81203568 | 12.57995293 |
discuss the position of the Br atoms with respect to the stability of the different conformers
The stability of the four conformers follows the order form most stable to least stable as ; Isomer 3 < Isomer 4 < Isomer 1 < Isomer 2. This means that the formation of Al-Br-Al three centre four electron bonds is favoured over Al-Cl-Al three centre four electron bonds. This could be because a Br atom will act as a better lewis base than a Cl atom because Cl is more electronegative and will hold onto its electrons more tightly making it harder to donate the lone pair to form the Al-X-Al three centre four electron bonds. As isomer 3 contains two Al-Br-Al three centre four electron bonds and isomer 4 contains one Al-Br-Al three centre four electron bond they are the two most stable confomers. The remaining two isomers with the higher energy Al-Cl-Al three centre four electron bonds form the least stable conformers.
Determining the dissociation energy for the lowest energy conformer in 2AlCl2Br
The lowest energy conformer of 2AlCl2Br is isomer 3.
Optimising AlCl2Br monomer
For the optimisation of the AlCl2Br monomer the same calculations weer carried out as before with the 4 conformers. Initially the lower level basis set 3-21G so used to obtain a good basic approximation of the structure, then after this was completed a mixed psueod-potential LanL2DZ was used on the heavier Br atoms and a higher basis sets 6-31G(d,p) was used the smaller Al and Cl atoms
Lower level basis set 3-21G optimisation
Log file : AlCl2Br_Monomer_OPT_3-21G.LOG
Gaussian calculation summary : AlCl2Br_Monomer_OPT_3-21G_Summary.LOG
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25681
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000039 0.001800 YES
RMS Displacement 0.000025 0.001200 YES
Predicted change in Energy=-3.947801D-10
Optimization completed.
-- Stationary point found.
This calculation successfully converged so now the mixture of PP with higher level basis set can be used.
Optimisation using PP and higher level basis set
Log file : AlCl2Br_Monomer_OPT_GEN.LOG
Gaussian calculation summary : AlCl2Br_Monomer_OPT_GEN_Summary.LOG
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25682
Item Value Threshold Converged?
Maximum Force 0.000005 0.000450 YES
RMS Force 0.000002 0.000300 YES
Maximum Displacement 0.000022 0.001800 YES
RMS Displacement 0.000012 0.001200 YES
Predicted change in Energy=-1.397811D-10
Optimization completed.
-- Stationary point found.
This calculation has successfully converged.
Energies
To find the bond dissociation energy of the the lowest energy isomer (Isomer 3) i have found the energy difference between that of isomer 3 and that of two monomers.
E(2AlCl2Br Isomer 3) = -2352.40630798 a.u.
E(AlCl2Br monomer) = -1176.19013697 a.u.
ΔE=E(2AlCl2Br Isomer 3)-2[E(AlCl2Br monomer)] = -0.02603404 a.u.
Association energy ( Kj/mol) = -68.3523772268 kj/mol
The dissociation energy is 68.3523772268 kj/mol, this means that energy is need to break the dimerised molecule into the two constituent monomers. Therefore Isomer 3 is more stable that the isolated monomers on account of the formation of the the Al-Br-Al three center four electron bridging bonds where a lone pair on each Br atom is donated to form two new Al-Br bonds with the electron deficient Al atoms.
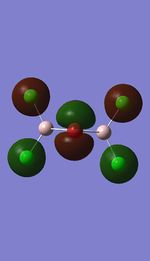
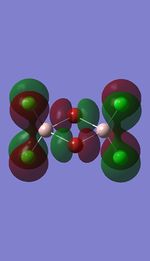
Molecular orbital analysis on the lowest energy conformer
Using the lowest energy conformer, Isomer 3, a population analysis was carried out on the chk.file of the mixed PP and higher basis set optimisation of isomer 3 . The job type was changed to Energy, input the keywords pop=full and pseudo=read, and selecting Full NBO.
Log file : Isomer3_MO_Analysis.LOG
Checkpoint file : Isomer3_MO_Analysis.fchk
Link to D-Space : https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/25707
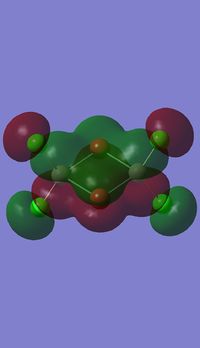
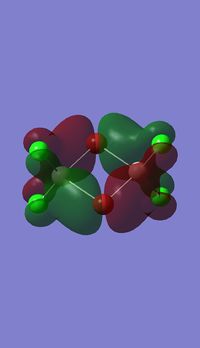
Molecular orbitals
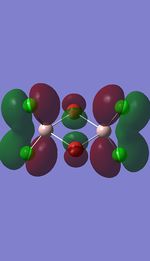
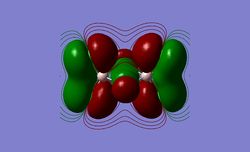
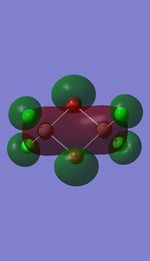
I have visualised all the occupied non-core MO's below from MO 31 upto Mo 54 (The HOMO).
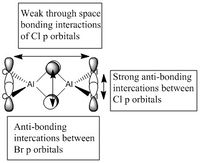
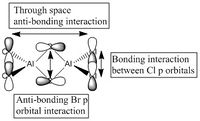
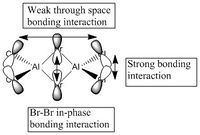
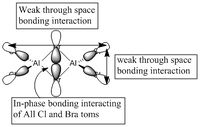
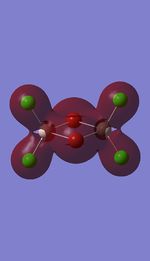
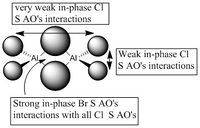
5 MOs interaction analysis
MO's 31,38 and 47 have shown bonding interaction that are not along the bonds that were initially drawn these were the Br-Br and Cl-Cl bonding interactions.Theses interactions indicate that the bonds initially drawn are not necessary the only existing bonds in the molecule and that they are shown this way to give us an initial understandable picture of the molecule. I now believe that there are Al-Al bonding interactions, and this can be supported by the MO analysis i carried out that showed there were much more interaction especially between the bridging Br atoms and Cl atoms which have brought the AL atoms close together which would allow for a Al-Al interaction. Where i to consider the optimised distance between the Al atoms in isomer 3 which is 3.47A, now compared to twice the Van Der Waal's radius of Al which is 3.68A. [2]. The optimised bond length is close to twice the Van Der Waal's radius which confirms that there must be a constructive Al-Al bonding interaction.
References
- ↑ Balázs Réffya, Mária Kolonits and Magdolna Hargittai, Journal of Molecular Structure, 1998, 445(1-3),139-148
- ↑ Manjeera Mantina, Adam C. Chamberlin, Rosendo Valero, Christopher J. Cramer, and Donald G. Truhlar, Consistent van der Waals Radii for the Whole Main Group, J. Phys. Chem. A 2009, 113,5806–5812