Rep:Mod:AliPage
Introduction
Page showing analysis of NH3, H2, N2, HCl and finally HBr for comparison. All optimization was completed using Gaussview 5.0.
NH3 Molecule
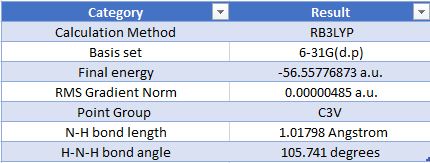
Geometric Information
Optimised Ammonia Molecule |
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
The optimization file is linked to here
Vibrational Modes
The following printscreen shows the display vibrations for the NH3 molecule:
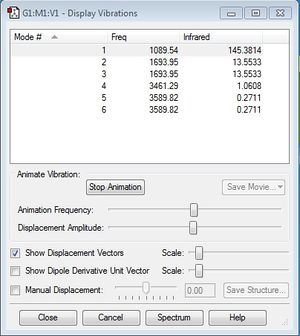
From the 3N-6 rule, we would expect (3*4) - 6 = 6 modes. Modes 2 and 3 are degenerate, and modes 5 and 6 are degenerate. Modes 1,2 and 3 are "bending" vibrations, while 3,4, and 5 are "bond stretch" vibrations. Mode 4 is highly symmetric. Mode 1 is known as the umbrella mode. I would only expect to see 2 peaks in the IR spectrum of gaseous ammonia. Of the six values given above, only the highest three intensities (145.38 and the two with 13.55) would be great enough to form visible peaks, and since two of the values (at 13.55 intensity) are degenerate they would combine to form one peak, resulting in two peaks in total.
Charge Analysis
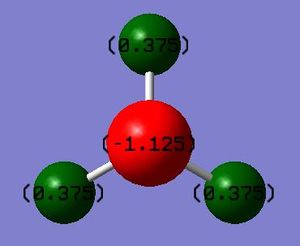
The charge on the N atom: -1.125 The charge on the H atoms: 0.375
I would expect the nitrogen to have a negative charge and for the hydrogens to have a positive charge because the nitrogen is more electronegative and therefore pulls the electrons in the covalent bonds more towards itself than the hydrogens, giving itself and partial negative charge and the hydrogens partial positive charges. The molecule is neutral overall, and therefore the charges added up come to zero.
H2 Molecule
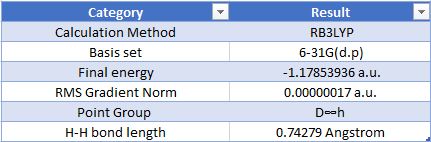
Geometric Information
Optimised Hydrogen Molecule |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
The optimization file is linked to here
Vibrational Modes
The following printscreen shows the display vibrations for the H2 molecule:
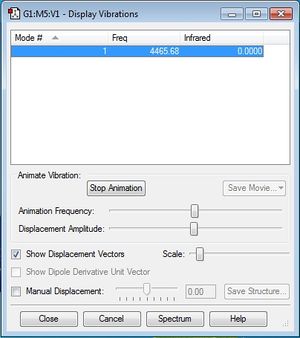
There was only one frequency and it was not negative, as seen here. Since the molecule is linear, following the 3N-5 rule, I would expect there to be (3*2)-5 = 1 frequency.
Charge Analysis
The charge on both hydrogen atoms is 0.00. This is to be expected, since both atoms have the same electronegativity, and so it is a purely covalent bond.
N2 Molecule
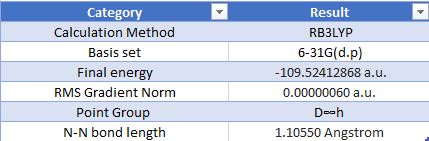
Geometric Information
Optimised Nitrogen Molecule |
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
The optimization file is linked to here
Vibrational Modes
The following printscreen shows the display vibrations for the N2 molecule:
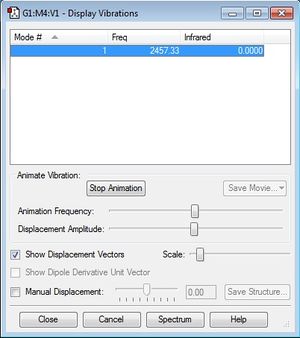
There was only one frequency and it was not negative, as seen here. Since the molecule is linear, following the 3N-5 rule, I would expect there to be (3*2)-5 = 1 frequency.
Charge Analysis
The charge on both nitrogen atoms is 0.00. This is to be expected, since both atoms have the same electronegativity, and so it is a purely covalent bond.
Haber-Bosch Reaction Energy Calculation
Calculating ΔE for the reaction N2 + 3H2 ==> 2NH3:
E(NH3)= -56.55776873 a.u. 2*E(NH3)= -113.1155375 a.u. E(N2)= -109.52412868 a.u. E(H2)= -1.17853936 a.u. 3*E(H2)= -3.53561808 a.u. ΔE=2*E(NH3)-[E(N2)+3*E(H2)]= -0.05579074 a.u. In kJ/mol this is (-0.05579074*2625.5) = -146.4785879 kJ/mol
The energy required to convert the gaseous reactants into ammonia is -146.479 kJ/mol. The ammonia is more stable, due to having a lower final energy than the reactants.
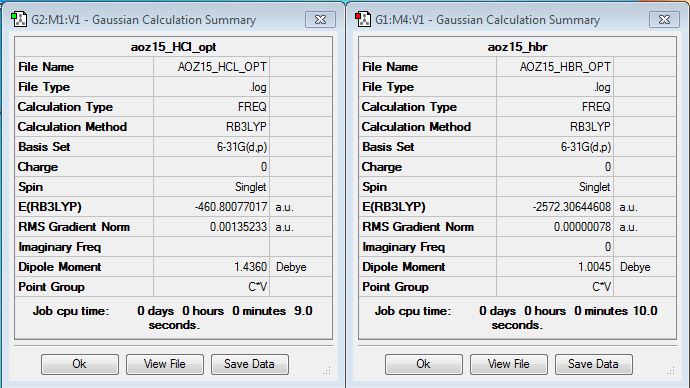
Project Molecule: HCl
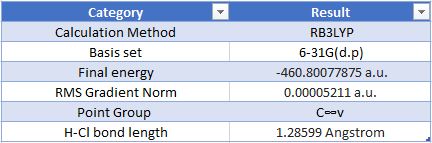
Geometric Information
Optimised HCl Molecule |
Item Value Threshold Converged? Maximum Force 0.000090 0.000450 YES RMS Force 0.000090 0.000300 YES Maximum Displacement 0.000139 0.001800 YES RMS Displacement 0.000197 0.001200 YES
The optimization file is linked to here
Vibrational Modes

The following printscreen shows the display vibrations for the HCl molecule:
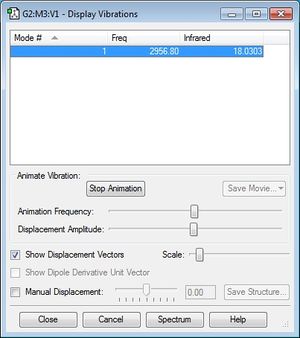
There was only one frequency and it was not negative, as seen here. Since the molecule is linear, following the 3N-5 rule, I would expect there to be (3*2)-5 = 1 frequency. This can be seen in the following IR Spectrumː
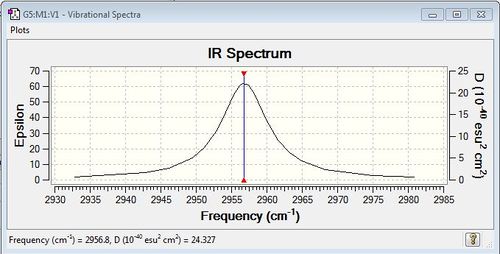
Charge Analysis
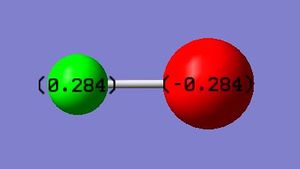
Charge on hydrogen: 0.284 Charge on chlorine: -0.284
The charges on the atoms can be explained by the fact that chlorine is more electronegative than hydrogen and so draws the electrons in the covalent bond towards itself more strongly, hence gaining a partial negative charge. The magnitude of the partial positive charge on the hydrogen is equal to that of the partial negative charge on the chlorine due to the molecule being neutral overall.
HCl Molecular Orbitals
Comparison Molecule: HBr
Geometric Information
Optimised HBr Molecule |
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000003 0.001800 YES
RMS Displacement 0.000004 0.001200 YES
The optimization file is linked to here
Vibrational Modes
The following printscreen shows the display vibrations for the HBr molecule:
There was only one frequency and it was not negative, as seen here. Since the molecule is linear, following the 3N-5 rule, I would expect there to be (3*2)-5 = 1 frequency.
Charge Analysis
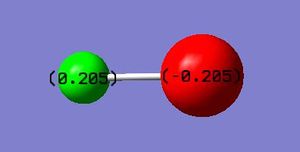
Charge on hydrogen: 0.205 Charge on bromine: -0.205
The charges on the atoms can be explained by the fact that bromine is more electronegative than hydrogen and so draws the electrons in the covalent bond towards itself more strongly, hence gaining a partial negative charge. The magnitude of the partial positive charge on the hydrogen is equal to that of the partial negative charge on the bromine due to the molecule being neutral overall.
Comparisonː HBr vs HCl
The two molecules were chosen for comparison because they are very similar, differing only in the halogen which is attached to the hydrogen. Bromine is the larger atom, but for this reason chlorine is more electronegative, as it has fewer orbitals to shield the nuclear charge from the electrons in a covalent bond. This explains why HCl has a larger dipole moment, as the electrons are more strongly attracted to the chlorine than they are to the bromine in HBr. It also explains the charge analysis results for the twoː both have a positively charged hydrogen and a negatively charged halogen, but the magnitude of the charges in greater in the HCl due to the greater difference in electronegativities between the atoms. Geometrically, both are linear and have no centre of inversion and therefore a C∞v point group. The energy of the HBr molecule is lower due to it being more stable; this is because of being less reactive due to having a lower dipole moment.