Rep:Mod:AlexanderGray
Organic Computational Labs Module
Calculations for Cyclopentadiene Dimers and their Hydrogenated Products
This part of the report uses ChemBio 3D to optimize complexes and determine the total energy of the molecule. The method used to optimize the molecules was a MM2 forcefield. The first comparison of energies was done between the 2 dimers of cyclopentadiene, it is known that cyclopentadiene takes the form of the endo dimer specifically over the exo dimer. By looking at the energies and hence the thermodynamic stability of the dimers it is possible to gain an insight into there formation.
1) Exo Dimer
This first molecule is the exo dimer of cyclopentadiene where MM2 minimization was used in order to obtain an energy minima.
Pentahelicene |
The table below shows the energy data obtained for the exo dimer.
------------MM2 Minimization------------ Note: All parameters used are finalized (Quality = 4). Iteration 76: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 1.2855 Bend: 20.5794 Stretch-Bend: -0.8381 Torsion: 7.6571 Non-1,4 VDW: -1.4171 1,4 VDW: 4.2322 Dipole/Dipole: 0.3776 Total Energy: 31.8766 kcal/mol Calculation completed ------------------------------------
2) Endo Dimer
This second molecule is the endo dimer of cyclopentadiene, again MM2 minimization was carried out.
Pentahelicene |
The minimization yielded the following results for the endo dimer:
------------MM2 Minimization------------ Note: All parameters used are finalized (Quality = 4). Iteration 103: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 1.2502 Bend: 20.8488 Stretch-Bend: -0.8357 Torsion: 9.5109 Non-1,4 VDW: -1.5455 1,4 VDW: 4.3210 Dipole/Dipole: 0.4477 Total Energy: 33.9975 kcal/mol Calculation completed ------------------------------------
By comparing the total energy of the two dimers it can be seen that the exo dimer is more stable by 2.1209 kcal/mol compared to the endo dimer, this is a significant difference. The majority of the energy difference observed here is due to torsion in the molecules as seen tables that sum to the total energy. This means that the reaction that forms cyclopentadiene is not controlled by only thermodynamics. It is believed therefore the the product formation is due to steric or orbital effects as it is known the endo dimer is in actuality favored, despite the fact that the exo dimer was calculated to be more energetically stable.
The endo dimer can be hydrogenated at either of the two double bonds, this would result in the two molecules seen below, the same method of optimization has been used here so that the total energies of each molecule can be compared.
3) Endo Hydrogenation (1)
Pentahelicene |
------------MM2 Minimization------------ Note: All parameters used are finalized (Quality = 4). Iteration 2: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 1.2782 Bend: 19.8555 Stretch-Bend: -0.8346 Torsion: 10.8110 Non-1,4 VDW: -1.2202 1,4 VDW: 5.6331 Dipole/Dipole: 0.1621 Total Energy: 35.6850 kcal/mol Calculation completed ------------------------------------
4) Endo Hydrogenation (2)
Pentahelicene |
------------MM2 Minimization------------ Note: All parameters used are finalized (Quality = 4). Iteration 153: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 1.0968 Bend: 14.5244 Stretch-Bend: -0.5493 Torsion: 12.4974 Non-1,4 VDW: -1.0692 1,4 VDW: 4.5112 Dipole/Dipole: 0.1406 Total Energy: 31.1520 kcal/mol Calculation completed ------------------------------------
Again, it is possible to compare the difference between the two total energies. The second hydrogenated molecule is more stable that first one by 4.533 kcal/mol, this means that it is the thermodynamic product of the hydrogenation reaction. It is possible to understand the different energies better by comparing the components that make up each total energy. The major contribution the large total energy difference between the two molecules appears to be the bending energy which has a difference of 5.3311 kcal/mol.
Comparison of Energies from Cyclopentadiene Dimers and There Hydrogenated Products
The table above has complicated the data from the above calculations so that the relative energy's of each component can be easily compared. It can be seen that between dimers or products most of the energy's are very similar and there are few differences between the two as would be expected.
This section of the report is looking at two isomers of an intermediate in the synthesis of taxol, the two isomers occur due to the possibility of rotation of the ketone group, it can either be orientated up or down on the ring. The first step at analyzing the two possible isomers was to construct them with the correct stereochemistry, these molecules where then optimized using a MM2 field and the energy was then calculated again using a MMFF94. The results of these calculations and the molecules constructed can be seen below.
Pentahelicene |
------------MM2 Minimization------------ Separating coincident atoms: H(26)-H(27) Separating coincident atoms: H(37)-H(39) Warning: Some parameters are guessed (Quality = 1). Iteration 228: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 2.7863 Bend: 16.5503 Stretch-Bend: 0.4310 Torsion: 18.2501 Non-1,4 VDW: -1.5648 1,4 VDW: 13.1116 Dipole/Dipole: -1.7249 Total Energy: 47.8396 kcal/mol Calculation completed ------------------------------------
------------MMFF94 Minimization------------ Iteration 58: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Final Energy: 70.5359 kcal/mol Calculation completed ------------------------------------

Pentahelicene |
------------MM2 Minimization------------ Separating coincident atoms: H(31)-H(32) Separating coincident atoms: H(35)-H(36) Warning: Some parameters are guessed (Quality = 1). Iteration 246: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 2.6228 Bend: 11.3382 Stretch-Bend: 0.3439 Torsion: 19.6639 Non-1,4 VDW: -2.1524 1,4 VDW: 12.8688 Dipole/Dipole: -2.0018 Total Energy: 42.6834 kcal/mol Calculation completed ------------------------------------
------------MMFF94 Minimization------------ Iteration 37: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Final Energy: 60.5719 kcal/mol Calculation completed ------------------------------------
By looking at the energies obtained by both calculations it can be seen that the second isomer is more stable than the first, the energy difference as calculated using the MM2 method is 5.1562 kcal/mol and the difference using the MMFF94 is 9.964 kcal/mol. It can therefore be said that the the intermediate with the ketone pointing in the same orientation as the neighboring hydrogen atoms is the one formed during the reaction. By looking at the composition of the energies calculated using the MM2 method the largest difference between the two is again in the bending component of the total energy. The key structural difference observed between the two isomers (apart form the inversion of the ketone) is that the 6 membered ring flips conformation, this is shown in the image below.
Comparison image showing ring flip:

Modelling Using Semi-empirical Molecular Orbital Theory.
This section of the report is looking at the diene shown below, the initial step where the same as above with the molecule being constructed in ChemBio 3D and then being optimized using the MM2 method, the results of which can be seen below:

------------MM2 Minimization------------ Warning: Some parameters are guessed (Quality = 1). Iteration 151: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 0.6183 Bend: 4.7355 Stretch-Bend: 0.0399 Torsion: 7.6600 Non-1,4 VDW: -1.0653 1,4 VDW: 5.7939 Dipole/Dipole: 0.1124 Total Energy: 17.8945 kcal/mol Calculation completed ------------------------------------
Pentahelicene |
The next step taken was to further optimize the molecule, this was done using the MOPAC method, this method takes into account any electronic effects that are not covered by the MM2 method. The result of the calculation can be seen below:
Mopac Job: AUX RM1 CHARGE=0 EF GNORM=0.100 SHIFT=80 Finished @ RMS Gradient = 0.09953 (< 0.10000) Heat of Formation = 22.82769 Kcal/Mol -----------------------------------------
The MOPAC calculation also involves the optimization of the molecule by compring this structure to the MM2 optimized one it can be seen that they have different geometries, this would be expected as the energies are different. The two different structures have been overlayed below to outline the key differences.
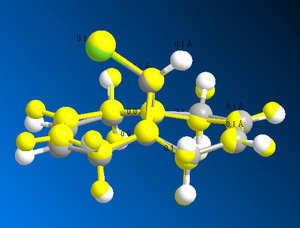
From the overlapped molecules it can be seen that the chlorine atom distorts the ring more in the highlighted MOPAC optimization. This means that there are orbital interaction involved that force the ring on the left hand side to bend downwards. This increases the strain on the molecule as so increases it energy, it it not possible to compare the energies of the two optimizations however as they are calculated through different methods.
The next part of the analysis involves modeling the molecular orbitals of the molecule, a 2D representation of the HOMO and LUMO can be seen below, the files for this calculation can be found here: DOI:10042/24374
One molecular orbital below the HOMO

The HOMO molecular orbital
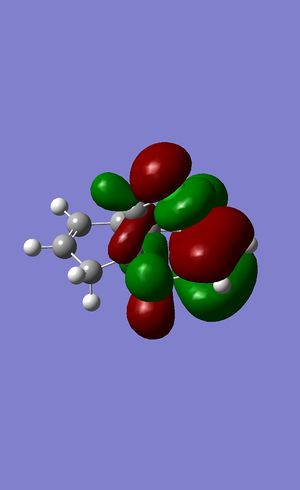
The LUMO molecular orbital
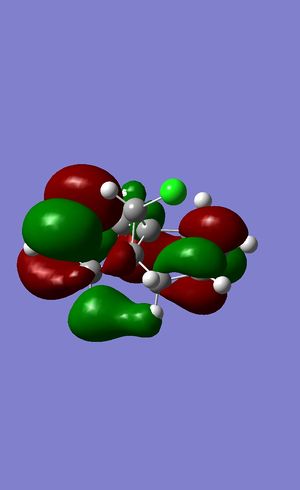
One above the LUMO molecular orbital

It can be seen that as expected these orbitals have a pane of symmetry along the molecule the chlorine atom has a large effect on the orbitals as the HOMO seems to center around it. The HOMO and LUMO are often looked at as they often give a lot of information about the bonding character of the molecule.
The image below shows the electrostatic potential surface for the molecule, it is symmetrical and has most of the negative charge based around the alkene bonds and the chlorine atom as would be expected.

The final part of the analysis of this molecule was a vibrational analysis, this was run in Gaussian on the previously optimized molecule. The resulting vibrations can be seen on the molecule below, to view the different vibration right click on the image and place the curser over "model", you can then select which vibration you wish to view, it also displays in that selection the frequency of the vibration.
Vibration |
Monosaccharide chemistry and the mechanism of glycosidation
The computational methods we have looked at before have been shown to provide a good understanding of intermediates in a reaction, this section will be looking at part of a glycosidation reaction which can be seen below:
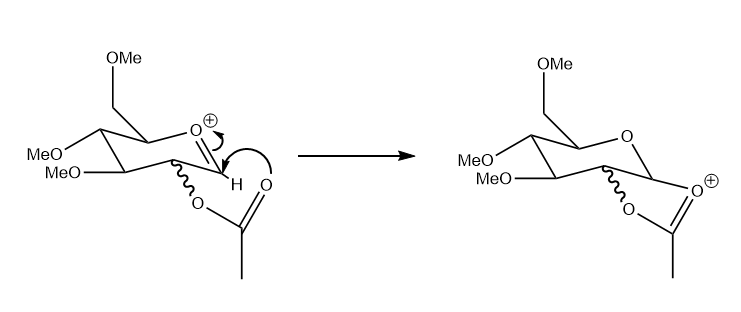
The first intermediate has 4 possible isomers, the optimizations for which can be seen below, the structures of each of isomer have been tabulated below for better comparison with there relevant energies.
Energy calculations for isomer 1
------------MM2 Minimization------------ Warning: Some parameters are guessed (Quality = 1). Iteration 2: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 2.4507 Bend: 11.5140 Stretch-Bend: 0.8750 Torsion: 1.4349 Non-1,4 VDW: 0.2620 1,4 VDW: 19.4581 Charge/Dipole: -28.6982 Dipole/Dipole: 5.2444 Total Energy: 12.5409 kcal/mol Calculation completed ------------------------------------
Mopac Job: AUX RM1 CHARGE=1 EF GNORM=0.100 SHIFT=80 Finished @ RMS Gradient = 0.09168 (< 0.10000) Heat of Formation = -70.04084 Kcal/Mol -----------------------------------------
Energy calculations for isomer 2
------------MM2 Minimization------------ Warning: Some parameters are guessed (Quality = 1). Iteration 2: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 2.4313 Bend: 11.7850 Stretch-Bend: 0.9826 Torsion: 1.4209 Non-1,4 VDW: 0.7701 1,4 VDW: 18.8357 Charge/Dipole: -27.0491 Dipole/Dipole: 5.9906 Total Energy: 15.1670 kcal/mol Calculation completed ------------------------------------
Mopac Job: AUX RM1 CHARGE=1 EF GNORM=0.100 SHIFT=80 Finished @ RMS Gradient = 0.09431 (< 0.10000) Heat of Formation = -79.27099 Kcal/Mol -----------------------------------------
Energy calculations for isomer 3
------------MM2 Minimization------------ Warning: Some parameters are guessed (Quality = 1). Iteration 447: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 2.2173 Bend: 10.6642 Stretch-Bend: 0.8233 Torsion: 0.6467 Non-1,4 VDW: -1.5632 1,4 VDW: 19.2644 Charge/Dipole: -15.9514 Dipole/Dipole: 3.8992 Total Energy: 20.0004 kcal/mol Calculation completed ------------------------------------
Mopac Job: AUX RM1 CHARGE=1 EF GNORM=0.100 SHIFT=80 Finished @ RMS Gradient = 0.07357 (< 0.10000) Heat of Formation = -65.36964 Kcal/Mol -----------------------------------------
Energy calculations for isomer 4
------------MM2 Minimization------------ Warning: Some parameters are guessed (Quality = 1). Iteration 2: Minimization terminated normally because the gradient norm is less than the minimum gradient norm Stretch: 2.2532 Bend: 11.1534 Stretch-Bend: 0.8510 Torsion: 1.1097 Non-1,4 VDW: -2.1220 1,4 VDW: 19.3508 Charge/Dipole: -11.5317 Dipole/Dipole: 3.8657 Total Energy: 24.9301 kcal/mol Calculation completed ------------------------------------
Mopac Job: AUX RM1 CHARGE=1 EF GNORM=0.100 SHIFT=80 Finished @ RMS Gradient = 0.08961 (< 0.10000) Heat of Formation = -62.83046 Kcal/Mol -----------------------------------------
This wiki page continues from the following link: [here]
| Isomer description | Visual of MM2 optimized isomer | Total energy of MM2 obtimzed isomer (Kcal/mol) | Visual of MOPAC optimized isomer | Heat of formation from MOPAC Kcal/mol | Distance between carbonyl oxygen and acyl carbon | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Axial and above the ring |
|
12.5409 |
|
-70.04084 | 2.00 | ||||||
| Axial and below the ring |
|
15.1670 |
|
-79.27099 | 1.457 | ||||||
| Equatorial and above the ring |
|
20.0004 |
|
-65.36964 | 2.869 | ||||||
| Equatorial and below the ring |
|
24.9301 |
|
-62.83046 | 2.869 |