Rep:Mod:Al7215 Yr2MO
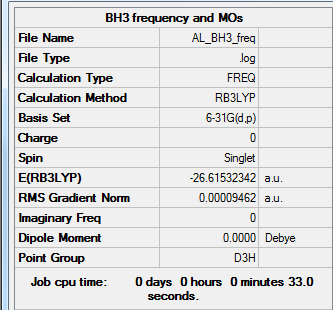
BH3 Molecule
Method: B3LYP/6-31G(d,p)
Item Table
Item Value Threshold Converged? Maximum Force 0.000189 0.000450 YES RMS Force 0.000095 0.000300 YES Maximum Displacement 0.000746 0.001800 YES RMS Displacement 0.000373 0.001200 YES
IR Frequencies
Low frequencies --- -0.2263 -0.1037 -0.0054 47.9770 49.0378 49.0383 Low frequencies --- 1163.7209 1213.6704 1213.6731
Frequency analysis log file:BH3_frequency.log
Optimised Borane Molecule |
IR Spectrum
| Wavenumber (cm-1) | Intensity (arbitrary units) | Symmetry | IR Active | Type of Vibration |
| 1164 | 92 | A2" | Yes | Out-of-plane bend |
| 1214 | 14 | E' | Very slight | In-plane bend |
| 1214 | 14 | E' | Very slight | In-plane bend |
| 2580 | 0 | A1' | No | Symmetric stretch |
| 2713 | 126 | E' | Yes | Asymmetric stretch |
| 2713 | 126 | E' | Yes | Asymmetric stretch |
There are less than 6 peaks as two of the asymmetric stretches at 1214 cm-1 and 2713 cm-1 have twofold degeneracy while the symmetric stretch at 2580 cm-1 is IR inactive and does not register a peak.
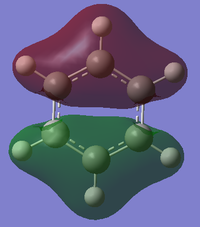
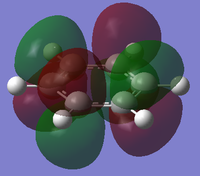
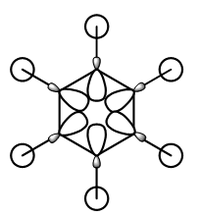
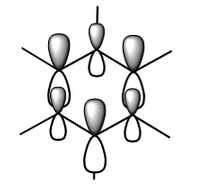
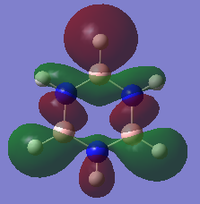
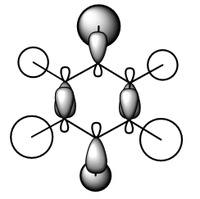
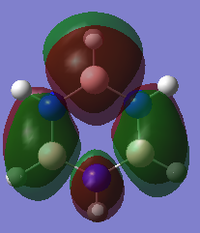
MO diagram for BH3
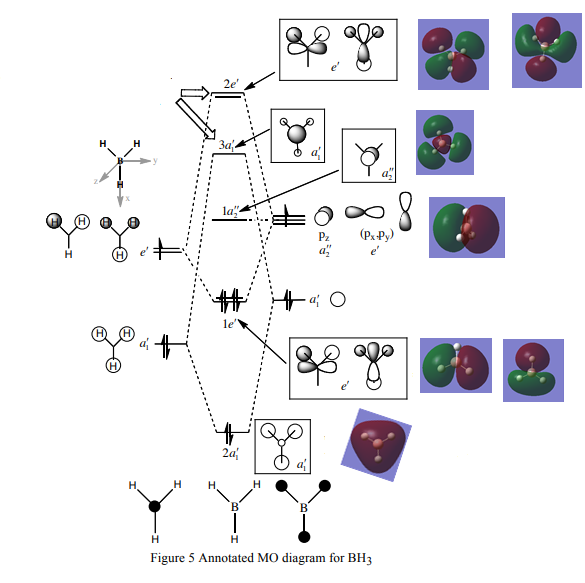
There is a slight difference in the LCAO and real MOs with A1' symmetry as the central lobe is much smaller and the adjacent green lobes are much larger for the real MO than the LCAO MO. Other than that, there are no significant differences between the real and LCAO MOs. This shows that the LCAO theory is a fairly good method to predict molecular orbitals for an unknown simple molecule without having to do complicated calculations.
Smf115 (talk) 23:59, 16 May 2018 (BST)Clearly presented MOs and good discussion of both the similarities and differences of the LCAOs and the MOs.
NH3 and NH3BH3 (Key Data)
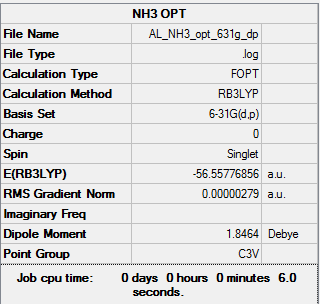
NH3 Molecule
Method: B3LYP/6-31G(d,p)
Item Table
Item Value Threshold Converged? Maximum Force 0.000005 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000014 0.001800 YES RMS Displacement 0.000009 0.001200 YES
IR Frequencies
Low frequencies --- -11.6527 -11.6490 -0.0045 0.0333 0.1312 25.5724 Low frequencies --- 1089.6616 1694.1736 1694.1736
Frequency analysis log file: NH3_frequency.log
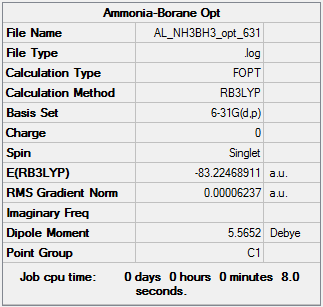
NH3BH3 Molecule
Method: B3LYP/6-31G(d,p)
Item Table
Item Value Threshold Converged? Maximum Force 0.000125 0.000450 YES RMS Force 0.000059 0.000300 YES Maximum Displacement 0.000614 0.001800 YES RMS Displacement 0.000313 0.001200 YES
IR Frequencies
Low frequencies --- -10.8522 0.0004 0.0009 0.0013 19.1618 42.3732 Low frequencies --- 266.1846 632.1840 638.2497
Frequency analysis log file: NH3BH3_frequency.log
Reaction Energies of Ammonia-Borane
Reaction: NH3 + BH3 -> NH3BH3
E(NH3) = -56.55776856 a.u. = -56.55778 a.u. (5 d.p.)
E(BH3)= -26.61532342 a.u. = -26.61532 a.u. (5 d.p.)
E(NH3BH3)= -83.22468911 a.u. = -83.22469 a.u. (5 d.p.)
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]= -0.05160 a.u. = -135 kJ/mol
The association energy of Ammonia-Borane is about -135 kJ/mol while its dissociation energy is 135 kJ/mol. Hence, B-N dative bond is weak as its dissociation energy is lower than that of a C-C bond, which is about 350 kJ/mol.
Smf115 (talk) 23:58, 16 May 2018 (BST)Good calculation but references required when quoting bond dissociation energies.
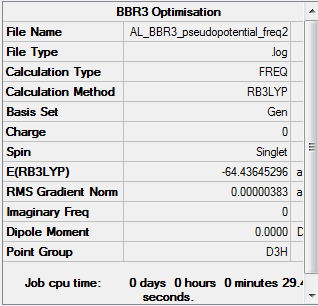
BBr3 Molecule
Method: B3LYP/6-31G(d,p) [Boron] & LanL2DZ [Bromine]
Item Table
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000036 0.001800 YES RMS Displacement 0.000018 0.001200 YES
Frequency analysis log file:BBr3_frequency.log
DSpace link: DOI:10042/202305
IR Frequencies
Low frequencies --- -0.0137 -0.0064 -0.0046 2.4315 2.4315 4.8421 Low frequencies --- 155.9631 155.9651 267.7052
Investigating Aromaticity (Mini-project)
Optimisation and Frequency Analysis of Benzene and Borazine
Benzene molecule
Method: B3LYP/6-31G(d,p)
Item Table
Item Value Threshold Converged? Maximum Force 0.000194 0.000450 YES RMS Force 0.000077 0.000300 YES Maximum Displacement 0.000824 0.001800 YES RMS Displacement 0.000289 0.001200 YES
IR Frequencies
Low frequencies --- -2.1456 -2.1456 -0.0088 -0.0040 -0.0040 10.4835 Low frequencies --- 413.9768 413.9768 621.1390
Frequency analysis log file:Benzene_frequency.log
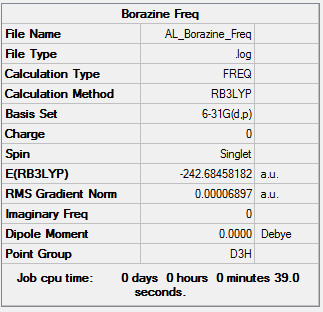
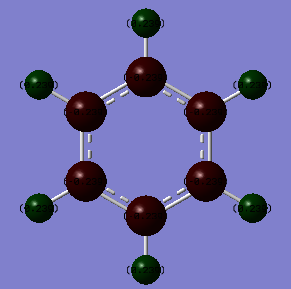
Borazine molecule
Method: B3LYP/6-31G(d,p)
Item Table
Item Value Threshold Converged? Maximum Force 0.000217 0.000450 YES RMS Force 0.000069 0.000300 YES Maximum Displacement 0.000333 0.001800 YES RMS Displacement 0.000106 0.001200 YES
IR Frequencies
Low frequencies --- -12.5651 -12.3512 -8.8739 -0.0100 0.0382 0.0785 Low frequencies --- 289.1192 289.1283 403.9050
Frequency analysis log file:Borazine_frequency.log
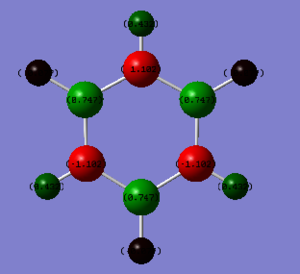
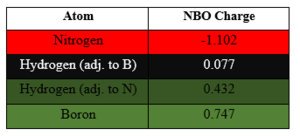
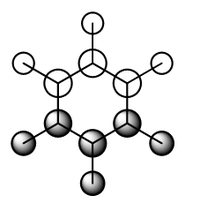
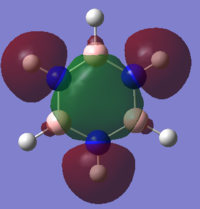
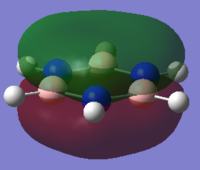
Charge Analysis of Benzene and Borazine
| Colour Range |
|---|
|
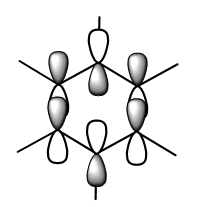
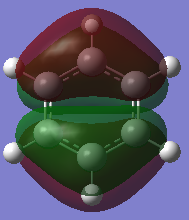
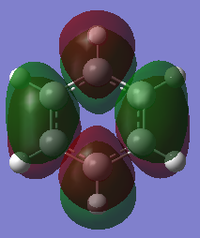
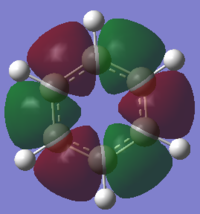
MO Analysis of Benzene
Smf115 (talk) 23:57, 16 May 2018 (BST)Great extra analysis of some of the MOs of both of the molecules and the corresponding LCAOs.
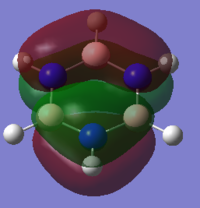
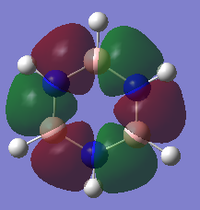
MO Analysis of Borazine
Comparing MOs of Benzene and Borazine
Concept of Aromaticity
The concept of aromaticity began with the isolation of benzene by Michael Faraday in 1825. Since then, many criteria or definitions for characterising aromaticity have been considered. The following lists a few of these criteria:[2]
1. Chemical behaviour: Electrophilic aromatic substitution- Such reactions is generally as it conserves the pi-electron structure of aromatic compounds;
2. Energetic: Large resonance energy- Aromatic compounds have enhanced stability compared to their olefinic analogues;
3. Structural properties: Bond length equalisation due to cyclic delocalisation;
4. Magnetic: “ring current” effects which include: analogous chemical shifts, large magnetic anisotropies and diamagnetic susceptibility exaltation
Overlapping pz AOs requires the planarity of a molecule. This, compared to those ascribed above, is not a good descriptor of aromaticity as lots of aromatic compounds are non-planar. A few good examples include: para- and meta- cyclophanes, pyrenophanes, or per-substituted derivatives of polyacenes.[3]
While aromaticity is not a property that is directly observable and lacks a well-founded physical basis, the computation of real MOs can help with the basic quantification and qualitative understanding of this fundamental chemical concept. For instance, real MOs can help to ascertain the high symmetry of aromatic compounds. This is certainly helpful as most archetypal aromatic compounds are highly symmetrical and possess degenerate HOMOs that are fully occupied, resulting in a closed-shell structure or have the same spin half-filled electronic structure.[4] (As seen earlier, benzene and borazine have HOMOs that are doubly degenerate)
Electron delocalisation is also an important aromatic descriptor that can be aided by localising real MOs and finding regions in which electron pairs are located. To date, the NBO analysis, used for calculation of charge distribution in benzene and borazine, is one of the most viable methods for localising bonds and lone pairs.[5]
Smf115 (talk) 23:56, 16 May 2018 (BST)Great discussion covering a range of points and with own literature referenced.
Smf115 (talk) 00:00, 17 May 2018 (BST)Overall a thorough report with a very good project section.
- ↑ Patricia A.Hunt, Problem Class 1 Answers, p.3
- ↑ Pure & Appl. Chem., Vol. 68, No. 2, pp. 209-218, 1996
- ↑ M. Palusiak, TM. Krygowski, Chemistry., 2007, 13, 7996-8006.
- ↑ F. Feixas, M. Eduard, P. Jordi, S. Miquel, Chem. Soc. Rev., 2015, 44, 6434
- ↑ F. Feixas, M. Eduard, P. Jordi, S. Miquel, Chem. Soc. Rev., 2015, 44, 6434