Rep:Mod:AS6115
Molecular Modelling
Introduction
This report documents the modelling of several molecules and the analysis of the data obtained from each model. All of the following molecule models have been created and optimized using the program GaussianView 5.0, which has also provided the data (e.g. bond lengths, bond angles, etc. ) on each of the molecules in the report.
NH3 molecule
Name: Ammonia
N-H bond length (Angstrom ) = 1.01798
H-N-H bond angle (degrees) = 104.741
Calculation method: RB3LYP
Basis set: 6-31G(d,p)
Final energy (au): -56.55776873
RMS gradient (au): 0.00000485
Point group: C3V
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES
Ammonia molecule |
Vibration analysis
According to the 3N-6 rule, Ammonia would be expected to have 6 vibration modes, there are two pairs of degenerate vibration modes as both have exactly the same frequency and therefore the same energy (these modes are vibrations 2 & 3 and 5 & 6). Vibration mode 1 is called the umbrella mode. The vibration mode 4 is highly symmetric stretching motion. Out of the six vibration modes of Ammonia three of them are stretching (modes 4,5,and 6) and the other three are bending motions (modes 1,2 and 3). Additionally based on this analysis the infra-red spectrum of gaseous Ammonia would be expected to have 4 bands experimentally. However practically it is more likely that only two bands will be observed as the vibration modes, as the vibration modes labelled 4, 5 and 6 (5 and 6 are degenerate) in the above Vibration display are shown to be very low in energy in the infra-red.
Charge analysis
Ammonia is a polar molecule, the Nitrogen atom of ammonia would be negatively charged as it is more electronegative than Hydrogen, so the electron of the N-H bond are closer to the Nitrogen atom; this is due to the greater effective nuclear charge of Nitrogen (as a result of the greater no. of protons/atomic number which outweighs the fact that Nitrogen has a larger atomic radius and a greater degree of electron shielding) compared to Hydrogen.
relative charges: Nitrogen=-1.25 au
Hydrogen=0.375 au
Ammonia can be synthesized by the reaction between Nitrogen and Hydrogen in the Haber-Bosch process.
N2 + 3H2 -> 2NH3
H2 molecule
Name: Hydrogen
H-H bond length (Angstrom)= 0.74279
Calculation method: RB3LYP
Basis set: 6-31G(d,p)
Final energy (au): -1.17853936
RMS gradient (au): 0.00000017
Point group: D∞h
Hydrogen molecule |
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
Hydrogen only has one vibration mode with a frequency of 4465.68cm-1
N2 molecule
Name: Nitrogen
N-N bond length (angstrom): 1.10550
Calculation method: RB3LYP
Basis set: 6-31G(d,p)
Final energy (au): -109.52412868
RMS gradient (au): 0.00000001
Point group: D∞h
Nitrogen molecule |
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Nitrogen only has one vibration mode with a frequency of 2457.33cm-1
Energy change for Haber-Bosch process: Haber-Bosch process N2 + 3H2 -> 2NH3
E(NH3)= -56.55776873 au
2*E(NH3)= -113.11553746 au
E(H2)= -1.17853936 au
3*E(H2)= -3.53561808 au
E(N2)= -109.52412868 au
ΔE= -0.0557907 au = -146.48 KJ/mol (to 2dp)
The energy change for this process is negative (exothermic reaction) therefore the product Ammonia is more stable than the gaseous sarting materials (Nitrogen and Hydrogen).
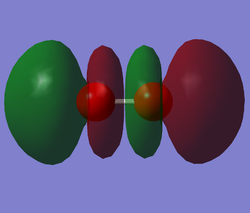
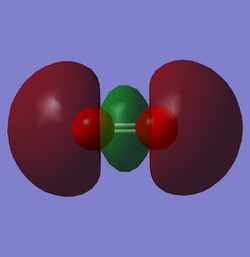
Molecular orbitals of Nitrogen
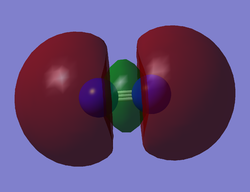
N2 HOMO
N2 LUMO
Project: O2 molecule
Summary Information
O-O bond length (Angstrom): 1.21576
Calculation method: RB3LYP
Basis set: 6-31G(d,p)
Final energy (au): -150.25742431
RMS gradient (au): 0.00015789
Point group: D∞h
Oxygen molecule |
Item Value Threshold Converged?
Maximum Force 0.000273 0.000450 YES
RMS Force 0.000273 0.000300 YES
Maximum Displacement 0.000167 0.001800 YES
RMS Displacement 0.000236 0.001200 YES
Charge analysis
Oxygen Molecule is homodinuclear molecule so there is no dipole present and thus the molecule is neutral. Also the molecular structure of Oxygen is linear.
Vibration Analysis
The Oxygen molecule has only one vibration mode; a symmetric stretch with a frequency of 1644.27 cm-1. Based on this model of the Oxygen molecule the experimental infra-red spectrum should consist of only a single band.
Molecular orbitals of Oxygen
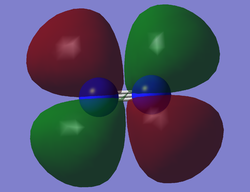
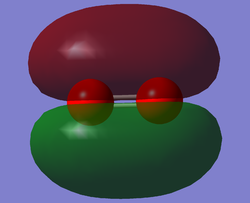
O2 HOMO
This is the 1π*g molecular orbital (MO). Two of the two 2p atomic orbitals (AOs) overlap in a sideways fashion to produce this MO, as well as the lower energy bonding MO. This MO is the HOMO (highest occupied molecular orbital). This is an antibonding MO and therefore the electrons which occupy this orbital have a destabilising effect on bonding.The orbital there are two nodal planes: one through the inter-nuclear axis and one perpendicular to the inter-nuclear axis through the middle of the O-O bond. There are two degenerate 1π*g MOs each of which are occupied singly by parallel spinning electrons.
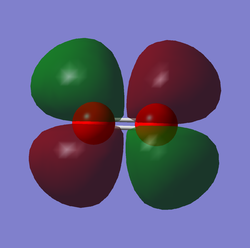
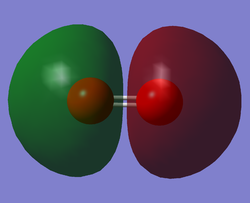
O2 LUMO
This is the 3б*u MO. This is the LUMO (Lowest unoccupied molecular orbital) and is formed by the linear combination of two 2p orbitals, one form each of the Oxygen atoms; the AOs approach along the inter-nuclear axis (i.e. head-on overlap).
O2 Sigma bonding molecular orbital
This is the 3бg MO, which is a bonding MO that is relatively high in energy and occupied by a pair of electrons. This MO is also formed by the linear combination of 2p AOs along the inter-nuclear axis which formed the LUMO (3б*u).
O2 Pi bonding molecular orbital
This MO is the 1πu; one of the 2p orbitals on each of the two oxygen atoms, overlap sideways to form this π bonding MO and a π antibonding MO. The MO is occupied by a pair of electrons. This is a bonding MO and is higher in energy than the 3бg MO.
O2 Sigma antibonding molecular orbital
This MO is the 2б*u; the atomic orbitals which linearly combine in order to form this molecular orbital are the 2s orbitals of each Oxygen atom in the molecule. This is an antibonding MO it has a nodal plane in the middle of the O-O bond separating the two lobes of the MO, and therefore is higher in energy than the 2s orbitals on oxygen from which it was formed. The MO is the 2б*u MO is occupied.