Rep:Mod:AM9315 Y2
EX3 Section
Ng611 (talk) 21:42, 20 May 2018 (BST) A lot of information here, but you were missing a number of key parts (complete frequency log files, your BH3 IR analysis, and interactive jmol images). There was also a lot of information in this section that we didn't ask for (e.g.: the graph showing energy vs optimisation step). Remember to read the script carefully and take care to include the information explicitly required of you.
BH3

Note that all BH3 molecules had bond angles of exactly 120.000o regardless of the level of optimisation and frequency.
BH3 energy and key information (3-21G)

Bond length for optimised 3-21G BH3 = 1.19453 A
Bond angle = 120.000o

Above, the log of information contains information such as the gradient of the slope for the energy vs distance graphs through the force items.
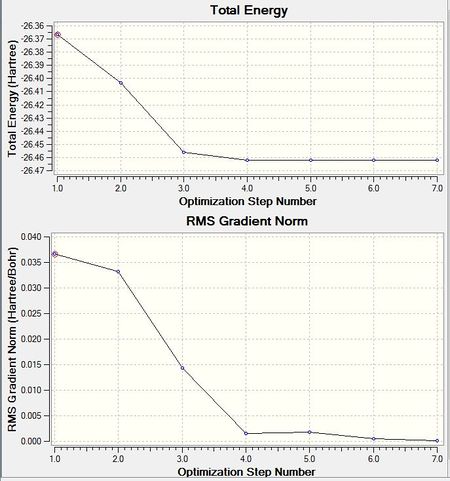
As is shown in the graph above, the optimisation allows the BH3 molecule to reach its equilibrium state, thus being able to stabilise the molecule. When nuclei and and electrons are not in equilibrium their mutual interactions, i.e. nuclear-nuclear repulsion, nuclear-electron attraction, electron-electron interactions are not stable, these interactions cause forces to be exerted on the nuclei and electrons making them shift into better positions. That is, the molecule will experience forces F(R) as long as a change in position (delta R) causes a change in energy, delta E(R).
BH3 energy and key information (6-31G)

Bond length = 1.19273 A
Note how the bond length changes simply by using a higher level basis set.
Bond angle = 120.000o
Also, note how the energy differs by using the a different basis method.
E(3-21G) = -26.61532342 a.u.
E(6-31G) = -26.61532342 a.u.
ΔE = 0.1530593 a.u. = 402 kJ/mol
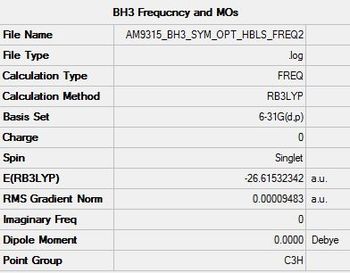
Note that the symmetry label after frequency analysis is C3H which cannot be explained as the bond angles are still exactly 120.000o.

Association energies: Ammonia and Borane
NH3


NH3BH3


E(NH3) = -56.55664134 a.u.
E(BH3) = -26.61532342 a.u.
E(NH3BH3) = -83.22469026 a.u.
ΔE = E(NH3BH3) - [E(NH3)+E(BH3)] = -0.0527255 a.u. = -138.4 kJ/mol.
Ng611 (talk) 21:40, 20 May 2018 (BST) Close, but off by a few Kj/mol. Remember also to report your units only to the nearest Kj/mol.
The dative BN bond is weaker than other Boron based bonds such as BCl which has a bond formation of -456 kJ/mol or a nitrogen based bond such as NCl which has a bond formation of -313kJ/mol.
Ng611 (talk) 21:40, 20 May 2018 (BST) Remember to cite your bond values (ideally from a textbook, databook, or paper).
MO and LCAO diagram for BH3

As is seen in the Figure above, there are no significant differences between the LCAO MOs (i.e. black and white images) and the and the real MOs. This demonstrates that qualitative MO theory is very accurate and useful in regards to building and describing Molecular Orbitals in a Chemistry sense.
BBr3


Ionic Liquids: Designer Solvents
[N(CH3)4]+

Dative N-C Bond length = 1.50936 A
Standard N-C Bond length = 1.50934(2-5) A
Tetrahedral Bond Angle = 109.47o




The first graph which shows the effects of optimisation against energy shows how the optimisation of the cation succeeds in achieving an energy minima where the cation is its most stable configuration. The frequency analysis further stabilizes the cation as can be shown when comparing the energy before and after frequency analysis.
Energy after optimisation = -214.18110186
Energy after optimisation and frequency analysis = -214.18128359

Charge of nitrogen = -0.295.
Charge of carbon atoms of methane = -0.483
Charge of hydrogen atoms of methane = 0.259
Ng611 (talk) 21:53, 20 May 2018 (BST) You needed to include a colour scale here. Your charge analysis was performed correctly though -- well done.
It is interesting to observe that the nitrogen (central species) has a higher charge, thus lower electron density surrounding it compared to the carbon (ligand). One would not expect that as nitrogen has an electronegativity of 3.04 and carbon has an electronegativity of 2.55, that the charge centered around the nitrogen. An explanation as to why this is not the case is that the fact that the molecule is [N(CH3)4]+, thus the one of the nitrogen bonds is dative meaning that a lone electron pair is being donated into the methyl group. This is shown by one bond being significantly shorter than the others. However, the Charge distribution shows that all carbon atoms have the same charge which would not be the case if one was receiving electron density. It could be that due to the effectiveness of nitrogen as a Lewis base i.e. a strong donor and the electronegativity difference in favour of nitrogen result in the electron density of the NC bond would be concentrated at the center of the bond. The carbon atoms would then also receive additional electron density from the methane groups thus making it the group with the highest charge density in this cation.
This theory slightly support the theory that in an ionic structure, the ligand would have the charge concentrated on the central atom (as shown below). The charge seems to represent the relative charge of the central species compared to its ligand and in this instance, appears to be valid as the charge distribution of the carbon ligands is more negative than that of the nitrogen central species. However, the image shown below is not entirely accurate as the electron density surrounding the nitrogen species. A more accurate image would rather show the positive charge for each individual hydrogen atom since this is really where the electron density is lowest, thus the charge value is the highest.
Ng611 (talk) 21:53, 20 May 2018 (BST) This is a good explanation, well done.

MO diagrams (LCAO and real)
Ng611 (talk) 21:55, 20 May 2018 (BST) Where are these??
[P(CH3)4]+

Ng611 (talk) 22:00, 20 May 2018 (BST) This is NMe4, not PMe4.
P-C Bond length = 1.816(69-72) A
Tetrahedral Bond Angle = 109.468o
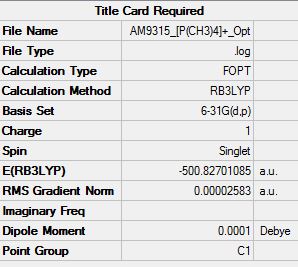



The first graph which shows the effects of optimisation against energy shows how the optimisation of the cation succeeds in achieving an energy minima where the cation is its most stable configuration. The frequency analysis further stabilizes the cation as can be shown when comparing the energy before and after frequency analysis.
Energy after optimisation = -500.82292956
Energy after optimisation and frequency analysis = -500.82699271

Charge of phosphorus= 1.667.
Charge of carbon atoms of methane = -1.050
Charge of hydrogen atoms of methane = 0.298
Unlike the nitrogen example, one would expect that phosphorous would have a higher charge as it is less electronegative than carbon thus one would expect that the electron density to be closer to the carbons of methane hence why they have a lower charge. The effect of this is that the positive charge is more likely to be found closer to the phosphorus, meaning this will more accurately represent what the theoretical image shown above. The fact that the phosphonium appears more ionic means that it can more effectivley couple to anionic liquids thus making it have a higher melting point compared to an equivalent ammonium salt e.g. tetramethlyl ammonium iodide has a mp of 280oC[1] and tetramethylphosphonium iodide has a melting point of around 325oC[2]. This is probably due to the fact that phosphorus is softer than nitrogen, thus forms stronger dative bonds, and as the charge is more dispersed, it means that it is a more effective cationic solvent.
Ng611 (talk) 22:00, 20 May 2018 (BST) Again, good discussion. Well done.
Ng611 (talk) 22:00, 20 May 2018 (BST) This report was missing a great deal of content (job information, fill log files, MO/FO analysis for NMe4/PMe4, to name a few). Your charge analysis was well explained and well performed, and if you had replicated this standard of work across the entire wiki, you would have a truly excellent report.
References
[1] E. Wait and H. M. Powell (1958). "The crystal and molecular structure of tetraethylammonium iodide." J. Chem. Soc. 1872-1875. [2] JPETAB Journal of Pharmacology and Experimental Therapeutics. (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21202) V.1- 1909/10- Volume(issue)/page/year: 25,315,1925
