Rep:Mod:AM8709mod3
Cope Rearrangement

The Cope reaction undergoes via [3,3] sigmatropic rearrangement mechanism. In this study the Cope rearrangement of 1,5-hexadiene was investigated. The reaction is sometimes considered to proceed through a diradical transition state.[1], but in this case it is assumed to be a concerted reaction. Because the Cope rearrangement of 1,5-hexadiene is currently accepted to go through a concerted pathway via an aromatic transition state.[2]
1,5-hexadiene
Optimisations for 1,5-hexadiene
For 1,5-hexadiene, it can adopt many conformations with low energy. Therefore, in this section the lowest energy conformation will be determined. Such conformations can be categorised into 2 groups: gauche and anti. First of all, the lowest energy gauche-conformation is going to be determined.
Optimisation for gauche-1,5-hexadiene
The structure of gauche-1,5-hexadiene was drawn and cleaned using the edit in Gaussview and was optimised using Hartree-Fock, HF, method with 3-21G basis set. The results from the optimisation of the first gauche-conformation or Gauche A shown as follows.
Gauche A

Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000695 0.001800 YES RMS Displacement 0.000214 0.001200 YES Predicted change in Energy=-1.830320D-08 Optimization completed. -- Stationary point found.

The optimisation was successfully achieved because all values of forces and distances are converged as well as the gradient shown in the summary result is less than 0.001.
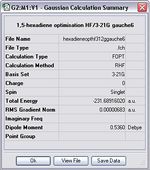
According to the summary table obtained from the optimisation, the energy of Gauche A is -231.68772 a.u.. Even though, the point group of the structure was not shown in the table, it can be figured out using Gaussview. Gauche A is classified into the C2 point group. Moreover, Gauche A is equivalent to "gauche1" shown in the Appendix 1 in The Cope Rearrangement Tutorial. According to theAppendix 1, "gauche1" is not the lowest energy conformation. This is due to the fact that the steric hindrance between two alkene substituents.
The results from the optimisation of the second gauche-conformation or Gauche B shown as follows.
Gauche B

Item Value Threshold Converged? Maximum Force 0.000014 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000695 0.001800 YES RMS Displacement 0.000214 0.001200 YES Predicted change in Energy=-1.830320D-08 Optimization completed. -- Stationary point found.
Like earlier the optimisation was successfully completed as the forces and distances are converged and the gradient is less than 0.001.
From the summary table, the energy of Gauche B is -231.68916 a.u.. Gauche B is in the C1 point group. In addition, Gauche B is equivalent to "gauche6" shown in the Appendix 1. With the same reason of the steric effect this conformation is still not the lowest energy gauche-conformation. According to the table shown in the Appendix 1, the lowest energy structure of the gauche-conformations is "gauche3" with the energy of -231.69266 a.u.. Interestingly, "gauche3" possesses even lower energy that all anti-conformations do. This is because of the attractive interaction between the π-orbital on the alkene and the vinyl proton within 1,5-hexadiene. [3]
Optimisation for anti-1,5-hexadiene
The structure of anti-1,5-hexadiene was drawn and cleaned using the edit in Gaussview and was optimised using HF/3-21G. The results from the optimisation of the first anti-conformation or Anti A shown as follows.
Anti A

Item Value Threshold Converged? Maximum Force 0.000089 0.000450 YES RMS Force 0.000022 0.000300 YES Maximum Displacement 0.000946 0.001800 YES RMS Displacement 0.000379 0.001200 YES Predicted change in Energy=-9.889015D-08 Optimization completed. -- Stationary point found.

All forces and distances are converged and the gradient is less than 0.001. These indicate that the optimisation was truly finished.
The energy of Anti A obtained from the optimisation is -231.69254 a.u.. Anti Ais in the Ci point group. Anti A is equivalent to "anti2" shown in the Appendix 1. This type of conformations is expected to posses a lower energy compared to the energy possessed by the gauche-conformations, because there is less steric crash between the two alkene substitutions within theanti-conformations than that for gauche-conformations. However, this is not true for the case of "gauche3" mentioned earlier. Moreover, once again the table in the appendix 1 indicates that the lowest energy structure among the anti-conformations is actually "anti1" not "anti2".
The results from the optimisation of the second anti-conformation or Anti B shown as follows.
Anti B

Item Value Threshold Converged? Maximum Force 0.000058 0.000450 YES RMS Force 0.000013 0.000300 YES Maximum Displacement 0.001664 0.001800 YES RMS Displacement 0.000580 0.001200 YES Predicted change in Energy=-2.791927D-08 Optimization completed. -- Stationary point found.

The forces and distances are converged with the gradient is less than 0.001. That is, the optimisation was successfully finished.
The energy of Anti B obtained from the optimisation is -231.68907 a.u.. Anti Bis categorised in the C2h point group. Anti B is matched to "anti3" shown in the appendix 1. This conformation unsurprisingly has higher energy than that "anti2" as the alkene groups within "anti3" point to the centre of the molecule, which causes steric repulsion. Meanwhile, the alkene groups within "anti2" point away from the centre causing less steric repulsion.
Reoptimisations
The four optimised conformations from HF/3-21G above are reoptimised with a better method, DFT/B3LYP/6-31G*. The results from the DFT approach are listed as follows.
Gauche A


Item Value Threshold Converged? Maximum Force 0.000020 0.000450 YES RMS Force 0.000009 0.000300 YES Maximum Displacement 0.000480 0.001800 YES RMS Displacement 0.000171 0.001200 YES Predicted change in Energy=-2.012605D-08 Optimization completed. -- Stationary point found.
Gauche B
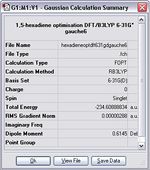
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000002 0.000300 YES Maximum Displacement 0.000291 0.001800 YES RMS Displacement 0.000073 0.001200 YES Predicted change in Energy=-1.337415D-09 Optimization completed. -- Stationary point found.
Anti A


Item Value Threshold Converged? Maximum Force 0.000111 0.000450 YES RMS Force 0.000036 0.000300 YES Maximum Displacement 0.001274 0.001800 YES RMS Displacement 0.000426 0.001200 YES Predicted change in Energy=-4.342873D-07 Optimization completed. -- Stationary point found.
Anti B


Item Value Threshold Converged? Maximum Force 0.000011 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.001169 0.001800 YES RMS Displacement 0.000398 0.001200 YES Predicted change in Energy=-5.258421D-09 Optimization completed. -- Stationary point found.
The reoptimisations above were successfully achieved as in each reoptimisation all forces and distances are converged as well as the gradient is less than 0.001.
The following table is a comparison between each optimisation for each conformation .
| Conformers | Point group | Jmol | Equivalent conformer in the appendix 1 (Energy/ Hartree) | Energy from HF/3-21G/ Hartree | Energy from DFT/B3LYP/ Hartree |
|---|---|---|---|---|---|
| Gauche A | C2 | gauche1 (-231.68772) | -231.68772 | -234.60788 | |
| Gauche B | C1 | gauche6 ( -231.68916) | -231.68916 | -234.60889 | |
| Anti A | Ci | anti2 ( -231.69254) | -231.69254 | -234.61171 | |
| Anti B | C2h | anti3 (-231.68907) | -231.68907 | -234.60962 |
From the table it can be seen that the energies of all the conformers obtained from the optimisations are identical to the energies mentioned in the appendix 1. The results from the HF/3-21G optimisations indicate that the lowest energy conformer is Anti A. The same indication was obtained form the DFT/B3LYP optimisations. However, the former shows that Anti B has higher energy than Gauche B. While the latter illustrates that Anti B possesses lower energy than Gauche B does, which is more reasonable as Anti B has lower steric hindrance. Despite the fact that the energies yielded from HF/3-21G and DFT/B3LYP indicate some differences, the symmetries of all the conformers are the same. The geometries from both methods are slightly different as shown in the table below.
| Conformers | Dihedral angles 2 vinyl groups (HF,DFT)/° | C-C bond length (HF,DFT) /Å | C-C bond length in the vinyl group (HF,DFT) /Å | C=C bond length (HF,DFT) /Å |
|---|---|---|---|---|
| Gauche A | (75.8,75.5) | (1.54,1.54) | (1.51,1.51) | (1.32,1.33) |
| Gauche B | (70.2,70.5) | (1.54,1.54) | (1.51,1.51) | (1.32,1.33) |
| Anti A | (179.9, 179.9 ) | (1.54,1.54) | (1.54,1.54) | (1.36,1.36) |
| Anti B | (179.9, 179.9 ) | (1.54,1.54) | (1.54,1.54) | (1.36,1.36) |
Frequency analysis for 1,5-hexadiene
In order to check whether the optimisation has reached the minimum energy the frequency calculation must be done in order to do so. If the frequencies obtained from such a calculation turn out to be positive, the optimised structure is at the minimum energy. While, if such frequencies are negative, the optimised structure is at the transition state.
6-31G* frequency calculation for Gauche A
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 93.0667 139.2699 145.7215
Red. masses -- 2.5534 1.7486 2.3740
Frc consts -- 0.0130 0.0200 0.0297
IR Inten -- 0.0063 0.3188 0.0359
. . .
4 5 6
A A A
Frequencies -- 261.0193 278.1199 486.2550
Red. masses -- 1.9345 2.5126 2.2152
Frc consts -- 0.0777 0.1145 0.3086
IR Inten -- 0.4610 0.3415 0.0929
. . .
7 8 9
A A A
Frequencies -- 527.1881 567.0142 624.2994
Red. masses -- 1.2400 1.3015 1.9478
Frc consts -- 0.2031 0.2465 0.4473
IR Inten -- 7.4569 7.4582 1.3648
. . .
10 11 12
A A A
Frequencies -- 800.7365 872.2326 929.7146
Red. masses -- 3.1215 1.9078 1.3456
Frc consts -- 1.1792 0.8551 0.6853
IR Inten -- 0.4899 0.0814 54.2037
. . .
13 14 15
A A A
Frequencies -- 933.6327 963.4567 1001.5842
Red. masses -- 1.3412 1.9486 1.5158
Frc consts -- 0.6888 1.0657 0.8959
IR Inten -- 20.3026 0.7905 0.0213
. . .
16 17 18
A A A
Frequencies -- 1013.2129 1039.2460 1045.1455
Red. masses -- 1.7038 1.0764 1.1711
Frc consts -- 1.0306 0.6850 0.7537
IR Inten -- 0.1466 15.8070 6.4126
. . .
19 20 21
A A A
Frequencies -- 1101.7962 1139.0516 1225.2579
Red. masses -- 1.5175 1.6226 1.1520
Frc consts -- 1.0854 1.2404 1.0189
IR Inten -- 2.1606 3.2301 1.4802
. . .
22 23 24
A A A
Frequencies -- 1286.6165 1343.1818 1347.9642
Red. masses -- 1.1775 1.2455 1.2584
Frc consts -- 1.1484 1.3239 1.3472
IR Inten -- 1.2274 2.3189 0.0653
. . .
25 26 27
A A A
Frequencies -- 1403.4755 1411.3572 1468.4162
Red. masses -- 1.4299 1.7201 1.2273
Frc consts -- 1.6595 2.0187 1.5592
IR Inten -- 7.2664 2.8056 0.4210
. . .
28 29 30
A A A
Frequencies -- 1474.9291 1505.0277 1508.9276
Red. masses -- 1.1553 1.1034 1.0717
Frc consts -- 1.4807 1.4726 1.4376
IR Inten -- 1.3776 4.5002 12.2819
. . .
31 32 33
A A A
Frequencies -- 1733.6348 1736.6284 3012.4402
Red. masses -- 4.4247 4.3554 1.0634
Frc consts -- 7.8351 7.7392 5.6856
IR Inten -- 10.7264 10.6057 18.6029
. . .
34 35 36
A A A
Frequencies -- 3018.3905 3036.8611 3042.6945
Red. masses -- 1.0636 1.0966 1.0980
Frc consts -- 5.7092 5.9587 5.9891
IR Inten -- 20.2185 31.5842 41.6430
. . .
37 38 39
A A A
Frequencies -- 3135.2102 3135.9567 3169.5887
Red. masses -- 1.0849 1.0849 1.0639
Frc consts -- 6.2832 6.2862 6.2975
IR Inten -- 56.3112 1.3823 7.1139
. . .
40 41 42
A A A
Frequencies -- 3169.8243 3244.9772 3245.4576
Red. masses -- 1.0638 1.1158 1.1155
Frc consts -- 6.2980 6.9223 6.9224
IR Inten -- 5.9427 35.7942 4.1367
. . .
------------------- - Thermochemistry - ------------------- Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
. . .
Zero-point correction= 0.142656 (Hartree/Particle) Thermal correction to Energy= 0.149775 Thermal correction to Enthalpy= 0.150719 Thermal correction to Gibbs Free Energy= 0.111817 Sum of electronic and zero-point Energies= -234.465220 Sum of electronic and thermal Energies= -234.458100 Sum of electronic and thermal Enthalpies= -234.457156 Sum of electronic and thermal Free Energies= -234.496058
6-31G* frequency calculation for Gauche B
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 87.0119 116.1867 144.7700
Red. masses -- 2.1458 2.6899 1.7911
Frc consts -- 0.0096 0.0214 0.0221
IR Inten -- 0.0282 0.1817 0.2450
...
4 5 6
A A A
Frequencies -- 248.7162 314.6579 420.7688
Red. masses -- 2.3135 1.9661 2.1103
Frc consts -- 0.0843 0.1147 0.2201
IR Inten -- 0.1026 0.1067 0.3098
...
7 8 9
A A A
Frequencies -- 550.9376 559.3003 635.2157
Red. masses -- 1.2587 1.9533 1.4947
Frc consts -- 0.2251 0.3600 0.3553
IR Inten -- 7.9925 1.2172 6.7803
...
10 11 12
A A A
Frequencies -- 822.2368 876.3466 932.5168
Red. masses -- 2.5439 1.8516 1.3471
Frc consts -- 1.0133 0.8378 0.6902
IR Inten -- 2.3636 0.4185 40.9814
...
13 14 15
A A A
Frequencies -- 936.2527 965.7986 975.7408
Red. masses -- 1.3436 1.6636 1.5420
Frc consts -- 0.6939 0.9143 0.8650
IR Inten -- 34.6550 1.1909 1.0126
...
16 17 18
A A A
Frequencies -- 1029.5346 1036.9689 1043.3780
Red. masses -- 1.9004 1.1097 1.0946
Frc consts -- 1.1868 0.7030 0.7021
IR Inten -- 0.2650 12.4375 10.0167
...
19 20 21
A A A
Frequencies -- 1116.1457 1148.2603 1250.1415
Red. masses -- 1.5134 1.7056 1.3634
Frc consts -- 1.1108 1.3250 1.2554
IR Inten -- 3.8872 3.3816 0.4439
...
22 23 24
A A A
Frequencies -- 1274.5196 1336.7616 1347.0635
Red. masses -- 1.1890 1.2319 1.2523
Frc consts -- 1.1380 1.2969 1.3389
IR Inten -- 1.3176 1.5607 1.4871
...
25 26 27
A A A
Frequencies -- 1374.5417 1410.8118 1474.1582
Red. masses -- 1.3544 1.5536 1.1842
Frc consts -- 1.5077 1.8219 1.5162
IR Inten -- 2.8860 6.0884 1.1462
...
28 29 30
A A A
Frequencies -- 1477.2123 1503.7600 1512.0445
Red. masses -- 1.2119 1.0766 1.1047
Frc consts -- 1.5581 1.4343 1.4880
IR Inten -- 1.0200 5.7344 7.8780
...
31 32 33
A A A
Frequencies -- 1731.3517 1736.2771 3005.4197
Red. masses -- 4.4930 4.3580 1.0630
Frc consts -- 7.9352 7.7406 5.6571
IR Inten -- 6.2100 11.6740 23.3276
...
34 35 36
A A A
Frequencies -- 3016.7521 3031.6369 3073.6436
Red. masses -- 1.0705 1.0947 1.0939
Frc consts -- 5.7403 5.9276 6.0890
IR Inten -- 28.2566 41.9609 22.2259
...
37 38 39
A A A
Frequencies -- 3133.7783 3134.2481 3153.9953
Red. masses -- 1.0851 1.0839 1.0661
Frc consts -- 6.2787 6.2737 6.2482
IR Inten -- 21.6516 36.1261 5.1688
...
40 41 42
A A A
Frequencies -- 3173.5176 3232.8254 3252.3344
Red. masses -- 1.0648 1.1151 1.1143
Frc consts -- 6.3185 6.8666 6.9448
IR Inten -- 9.7720 21.8817 17.1666
...
------------------- - Thermochemistry - ------------------- Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
...
Zero-point correction= 0.142608 (Hartree/Particle) Thermal correction to Energy= 0.149784 Thermal correction to Enthalpy= 0.150728 Thermal correction to Gibbs Free Energy= 0.111521 Sum of electronic and zero-point Energies= -234.466281 Sum of electronic and thermal Energies= -234.459104 Sum of electronic and thermal Enthalpies= -234.458160 Sum of electronic and thermal Free Energies= -234.497367
6-31G* frequency calculation for Anti A
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 74.3655 81.0728 121.4785
Red. masses -- 2.7340 2.6613 2.4734
Frc consts -- 0.0089 0.0103 0.0215
IR Inten -- 0.0195 0.1177 0.0000
. . .
4 5 6
A A A
Frequencies -- 220.7002 348.9083 394.4247
Red. masses -- 1.7643 2.4933 1.9819
Frc consts -- 0.0506 0.1788 0.1817
IR Inten -- 0.1570 0.0000 0.0000
. . .
7 8 9
A A A
Frequencies -- 462.2024 625.6843 669.4889
Red. masses -- 1.9605 1.5556 1.4846
Frc consts -- 0.2468 0.3588 0.3920
IR Inten -- 2.9014 0.0000 20.0120
. . .
10 11 12
A A A
Frequencies -- 788.2458 937.9559 938.3582
Red. masses -- 1.2173 2.0117 1.3478
Frc consts -- 0.4456 1.0428 0.6992
IR Inten -- 4.0393 11.4681 0.0000
. . .
13 14 15
A A A
Frequencies -- 939.8703 941.3636 1002.0324
Red. masses -- 1.4197 1.4208 1.8523
Frc consts -- 0.7389 0.7418 1.0958
IR Inten -- 62.1337 0.0001 0.0000
. . .
16 17 18
A A A
Frequencies -- 1033.9698 1035.8643 1042.6213
Red. masses -- 2.4890 1.0875 1.3220
Frc consts -- 1.5678 0.6875 0.8467
IR Inten -- 0.0000 19.7209 0.0000
. . .
19 20 21
A A A
Frequencies -- 1067.9598 1203.1653 1250.5080
Red. masses -- 1.3457 2.0974 1.4153
Frc consts -- 0.9043 1.7889 1.3040
IR Inten -- 9.6045 0.0000 0.5794
. . .
22 23 24
A A A
Frequencies -- 1289.0987 1323.1857 1338.6630
Red. masses -- 1.2803 1.1079 1.2609
Frc consts -- 1.2536 1.1428 1.3313
IR Inten -- 6.4718 0.0000 0.0000
. . .
25 26 27
A A A
Frequencies -- 1342.6236 1384.5208 1473.7191
Red. masses -- 1.2413 1.4055 1.1818
Frc consts -- 1.3184 1.5874 1.5122
IR Inten -- 1.3955 0.0000 0.0000
. . .
28 29 30
A A A
Frequencies -- 1476.1209 1509.2312 1523.6874
Red. masses -- 1.1825 1.1099 1.1069
Frc consts -- 1.5180 1.4895 1.5140
IR Inten -- 1.5095 0.0000 5.6261
. . .
31 32 33
A A A
Frequencies -- 1730.9646 1734.2014 3022.3135
Red. masses -- 4.4522 4.5018 1.0618
Frc consts -- 7.8597 7.9769 5.7145
IR Inten -- 0.0001 18.1168 0.0001
. . .
34 35 36
A A A
Frequencies -- 3031.8792 3060.4786 3080.4446
Red. masses -- 1.0612 1.0984 1.1026
Frc consts -- 5.7476 6.0616 6.1645
IR Inten -- 53.4689 0.0000 35.7410
. . .
37 38 39
A A A
Frequencies -- 3135.8095 3136.9078 3155.4421
Red. masses -- 1.0835 1.0834 1.0662
Frc consts -- 6.2771 6.2814 6.2548
IR Inten -- 0.0001 56.3037 14.6976
. . .
40 41 42
A A A
Frequencies -- 3155.6993 3233.9747 3234.0019
Red. masses -- 1.0665 1.1155 1.1155
Frc consts -- 6.2574 6.8737 6.8740
IR Inten -- 0.0095 0.1761 45.2738
. . .
------------------- - Thermochemistry - ------------------- Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
. . .
Zero-point correction= 0.142507 (Hartree/Particle) Thermal correction to Energy= 0.149853 Thermal correction to Enthalpy= 0.150797 Thermal correction to Gibbs Free Energy= 0.110935 Sum of electronic and zero-point Energies= -234.469203 Sum of electronic and thermal Energies= -234.461857 Sum of electronic and thermal Enthalpies= -234.460913 Sum of electronic and thermal Free Energies= -234.500775
6-31G* frequency calculation for Anti B
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 74.2787 150.7776 179.2687
Red. masses -- 2.9349 1.6843 1.5186
Frc consts -- 0.0095 0.0226 0.0288
IR Inten -- 0.0708 0.1168 0.0005
. . .
4 5 6
A A A
Frequencies -- 180.0759 264.7150 513.9725
Red. masses -- 2.8598 3.6233 2.5703
Frc consts -- 0.0546 0.1496 0.4000
IR Inten -- 0.7784 0.0000 0.1252
. . .
7 8 9
A A A
Frequencies -- 564.3383 569.4837 572.0085
Red. masses -- 1.2566 1.1841 2.6291
Frc consts -- 0.2358 0.2263 0.5068
IR Inten -- 0.0000 19.8114 0.0001
. . .
10 11 12
A A A
Frequencies -- 798.7029 915.2175 927.9041
Red. masses -- 1.2821 2.9235 2.4172
Frc consts -- 0.4819 1.4428 1.2262
IR Inten -- 0.1463 0.0000 2.1398
. . .
13 14 15
A A A
Frequencies -- 931.0787 933.1322 1007.7240
Red. masses -- 1.3273 1.3535 1.6898
Frc consts -- 0.6779 0.6944 1.0111
IR Inten -- 0.0000 71.6681 0.0000
. . .
16 17 18
A A A
Frequencies -- 1041.6587 1042.6726 1069.1188
Red. masses -- 1.0685 1.0620 1.9174
Frc consts -- 0.6831 0.6803 1.2913
IR Inten -- 19.0160 0.0000 0.0001
. . .
19 20 21
A A A
Frequencies -- 1078.6654 1153.3424 1211.5418
Red. masses -- 1.2764 1.8924 1.1529
Frc consts -- 0.8750 1.4831 0.9970
IR Inten -- 8.6684 0.0000 1.3029
. . .
22 23 24
A A A
Frequencies -- 1321.7706 1324.3868 1348.1118
Red. masses -- 1.6337 1.1011 1.2377
Frc consts -- 1.6816 1.1379 1.3253
IR Inten -- 2.0547 0.0001 5.7166
. . .
25 26 27
A A A
Frequencies -- 1348.8026 1431.0919 1471.6431
Red. masses -- 1.2580 1.7912 1.2012
Frc consts -- 1.3484 2.1614 1.5327
IR Inten -- 0.0000 0.0000 3.7734
. . .
28 29 30
A A A
Frequencies -- 1475.8702 1509.0873 1512.4757
Red. masses -- 1.1885 1.0733 1.0946
Frc consts -- 1.5253 1.4401 1.4753
IR Inten -- 0.0000 0.0000 10.0273
. . .
31 32 33
A A A
Frequencies -- 1729.8832 1736.9791 3011.1329
Red. masses -- 4.2095 4.3929 1.0597
Frc consts -- 7.4219 7.8089 5.6609
IR Inten -- 33.5729 0.0000 0.0000
. . .
34 35 36
A A A
Frequencies -- 3018.4735 3028.0162 3048.4927
Red. masses -- 1.0600 1.0989 1.1020
Frc consts -- 5.6901 5.9365 6.0340
IR Inten -- 40.9727 0.0000 49.2310
. . .
37 38 39
A A A
Frequencies -- 3139.8976 3140.4257 3168.8903
Red. masses -- 1.0848 1.0842 1.0645
Frc consts -- 6.3013 6.3002 6.2982
IR Inten -- 56.5225 0.0008 12.9882
. . .
40 41 42
A A A
Frequencies -- 3168.9245 3241.7141 3241.8713
Red. masses -- 1.0646 1.1158 1.1156
Frc consts -- 6.2989 6.9086 6.9079
IR Inten -- 0.0590 46.4743 0.0117
. . .
------------------- - Thermochemistry - ------------------- Temperature 298.150 Kelvin. Pressure 1.00000 Atm.
. . .
Zero-point correction= 0.142608 (Hartree/Particle) Thermal correction to Energy= 0.149802 Thermal correction to Enthalpy= 0.150746 Thermal correction to Gibbs Free Energy= 0.111556 Sum of electronic and zero-point Energies= -234.467014 Sum of electronic and thermal Energies= -234.459821 Sum of electronic and thermal Enthalpies= -234.458877 Sum of electronic and thermal Free Energies= -234.498066
According to the data obtained from the frequency calculations listed above, all optimised structures provide real positive frequencies. That is, such structures were optimised to their minimum energies.
Now the Thermochemistry results of Anti A will be considered and analysed. The table concluding the energies from the frequency calculations at 298.15 K and 1t 0 K is shown below.
| Energies | At 298.15 K | At 0 K | Notes |
|---|---|---|---|
| Sum of electronic and zero-point Energies | -234.469203 | -234.469204 | The potential electronic energy at 0 K with the zero-point energy (E = Eelec + EZP) |
| Sum of electronic and thermal Energies | -234.461857 | -234.469204 | The energy with the translational, rotational, and vibrational energies at 298.15 K and 1 atm (E = Eelec + Etrans + Erot + Evib) |
| Sum of electronic and thermal Enthalpies | -234.460913 | -234.469204 | An additional correction for room temperature (H = E + RT) |
| Sum of electronic and thermal Free Energies | -234.500775 | -234.469204 | The entropic contribution to the free energy (G = H - TS) |
It is obvious to see that the values at 0 K are all the same. This is because the values were calculated at 0 K as a result all molecules make no thermal contribution, i.e. such molecules are at the ground state. Therefore, the electronic energy at 0 K is equal to -234.46904 Hartree. Once the temperature rises to 298.15 K, unsurprisingly the value of the sum of electronic and zero-point energies remains that same since such a value is the value for at 0 K, although it was calculated at 298.15 K. On the other hand, the other energies change as the temperature rises, or in the other word the energies are temperature dependent (as can be seen from the equations in the table). The sum of electronic and thermal energies increases when the temperature rises because the molecules start to move, vibrate, and rotate. These increase the thermal energies. Likewise, the value of the thermal enthalpies get larger at 298.15 with the same reason. However, the thermal free energies drop as the temperature increases because of the fact that the disorder or degree of freedom of the molecules soars with the temperature (-TS). As a result from the equation G = H - TS, the free energies decrease as the temperature gets higher.
Transition states
C3H5 fragment
Boat and chair transition states were drawn by combining two fragments of C3H5. Therefore. it is important to draw a C3H5 fragment in the first place. The structure of C3H5 was optimised using HF/3-21G.

Item Value Threshold Converged? Maximum Force 0.000057 0.000450 YES RMS Force 0.000021 0.000300 YES Maximum Displacement 0.000694 0.001800 YES RMS Displacement 0.000312 0.001200 YES Predicted change in Energy=-7.410413D-08 Optimization completed. -- Stationary point found.
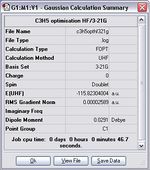
The fragment was completely optimised since the values shown above are converge. Once this fragment was optimised, the information reveals that the fragment has C2v symmetry and the structure also shows that the C-C bond lengths within the fragment are about 1.39 Å which are in between the normal C-C bond length and he normal C=C bond length. That is, this fragment shows the resonance character. After that two of the fragment were arranged to construct the chair and boat transition states of the Cope rearrangement, which will be shown in the next section.
Chair transition state
Two fragments of the optimised C3H5 were arranged to a chair structure. The distances between the terminal carbon atoms were set to about 2 Å as shown in the figure below.
Chair |
Optimisations
In this study two methods will be used in order to optimise the chair transition state drawn earlier. The first method (Method 1) is to optimise the transition states to a TS (Berny) by calculating the Hessian force constants once with the additional keyword Opt=NoEigen using the Opt+Freq option. The keywordOpt=NoEigenprevents the calculation from crashing if more than one imaginary frequency is found during the calculation. The second method (Method 2) is to use the frozen coordinate method.
Method 1
The results from the first method are shown as follows.
Item Value Threshold Converged? Maximum Force 0.000032 0.000450 YES RMS Force 0.000006 0.000300 YES Maximum Displacement 0.000308 0.001800 YES RMS Displacement 0.000069 0.001200 YES Predicted change in Energy=-1.476338D-08 Optimization completed. -- Stationary point found.
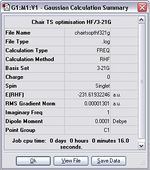
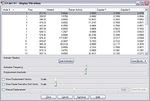
The calculation shows a negative frequency at -818 cm-1. This frequency corresponds to the vibration between the two C3H5 fragments at the transition state (click at the image below to illustrate the vibration).

The vibration illustrates that two terminal carbon atoms move apart from each other while the other two terminal carbon atoms move towards each other. The bond elongation shows the bond breaking and the bond shortening indicates the bond forming during the Cope rearrangement. In addition the energy and symmetry of this species are -231.61932 a.u. and C2h, which are matched to the data provided in the Appendix 2. The comparison between the values obtained from the calculation and the values given in the Appendix 2 is shown in the following table.
| Source | Electronic energy/ Hartree | Sum of electronic and zero-point Energies at 0 K/ Hartree |
|---|---|---|
| Frequency calculation | -231.61932 | -231.46670 |
| Appendix 2 | -231.619322 | -231.466705 |
Method 2
In order to set up the molecule for the frozen coordinate method, first the distances between the two terminus of the two fragments were set to 2.2 Å. The molecule was then optimised to the minimum using HF/3-21G.
The optimised structure was edited by remove the constrain from the distances between the two fragments and the structure was then reoptimised using the Opt+Freq option. The results from the frozen method are shown as follows.
Item Value Threshold Converged? Maximum Force 0.000039 0.000450 YES RMS Force 0.000014 0.000300 YES Maximum Displacement 0.000936 0.001800 YES RMS Displacement 0.000175 0.001200 YES Predicted change in Energy=-3.059443D-07 Optimization completed. -- Stationary point found.
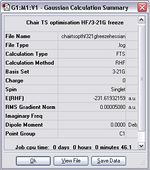
The energy and symmetry of this species are -231.61932 a.u. and C2h, which are matched to the results from Method 1 and the data provided in the Appendix 2.
Boat transitions state
Two fragments of the optimised C3H5 were arranged to a boat structure. The distances between the terminal carbon atoms were set to about 2.2 Å as shown in the figure below.
Chair |
Optimisations
For boat transition state QST2 methods will be used in order to optimise the transition state drawn earlier. Using this approach the reactant and product needed to specified in order to allow Gaussian to interpolate between the reactant and product so as to predict the transition state. Consequently, the reactants and products must bed numbered in the same way. Moreover, the geometries of the reactant and product must be adjusted as follows to prevent the failure of the optimisation. The optimisation was done using HF/3-21G.
| Reactant | Product |
QST2 method
The results from the QST2 method method are shown as follows.
Item Value Threshold Converged? Maximum Force 0.000166 0.000450 YES RMS Force 0.000037 0.000300 YES Maximum Displacement 0.000600 0.001800 YES RMS Displacement 0.000152 0.001200 YES Predicted change in Energy=-2.080014D-07 Optimization completed. -- Stationary point found.
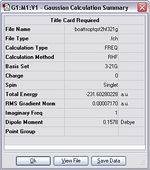

The vibration illustrates the asymmetric stretches between the two fragments. Theses stretches are the evidence for the bond breaking and bond forming during the rearrangement. In addition the energy and symmetry of this transition state are -231.60280 a.u. and C2v, which correspond to the data in the Appendix 2. The comparison between the values obtained from the calculation and the values given in the Appendix 2 is shown in the following table.
| Source | Electronic energy/ Hartree | Sum of electronic and zero-point Energies at 0 K/ Hartree |
|---|---|---|
| Frequency calculation | -231.60280 | -231.45093 |
| Appendix 2 | -231.602802 | -231.450929 |
The following table illustrates the comparison between energies of chair transition state and that for boat transition state.
| TS | Electronic energy/ Hartree | Sum of electronic and zero-point Energies at 0 K/ Hartree |
|---|---|---|
| Chair TS | -231.61932 | -231.46670 |
| Boat TS | -231.60280 | -231.45093 |
| differences/ Hartree | 0.01652 | 0.01577 |
As per the table, it can be seen that the Chair TS is more stable than the Boat as the former possesses a lower energy than the latter.
Intrinsic reaction coordinate (IRC)
Intrinsic Reaction Coordinate or IRC allows us to follow the minimum energy path from a transition state down to the local minimum on a potential energy surface. That is, the conformations for the reactant and product can be determined from such a method. The IRC generates sets of points by taking small geometry steps in the direction where the gradient of the energy surface is steepest.
IRC for chair transition state
DOI:10042/to-11625 Unfortunately the result obtained form the IRC method with the Hessian force constant once calculated indicates that the conformation of the 1,5-hexadiene was not at the minimum of the energy surface (-213.61932 a.u.).
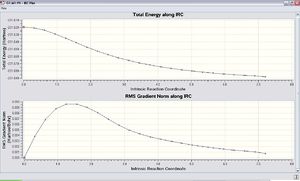

In order to solve this problem,The IRC approach was done by the Hessian force constant was calculated every time along the computation with the number of steps equals to 100. The result is shown as follows. DOI:10042/to-11626
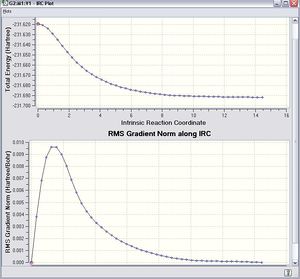
This time the conformation of the 1,5-hexadiene reached the minimum energy (-231.69165 a.u.). Once this energy was compared with the one in the table in the Appendix 1, it is found that the conformation at the minimum energy point is gauche2.
IRC for boat transition state
Like the chair transition state, the result obtained form the IRC method with the Hessian force constant once calculated indicates that the conformation of the 1,5-hexadiene was not at the minimum of the energy surface (-213.61168 a.u.).
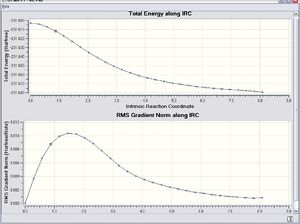
In order to solve this problem,The IRC approach was done by the Hessian force constant was calculated every time along the computation with the number of steps equals to 100. The result is shown as follows.
DOI:10042/to-11628
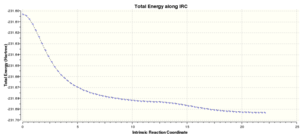
This time the conformation of the 1,5-hexadiene reached the minimum energy (-231.69266 a.u.). Once this energy was compared with the one in the table in the Appendix 1, it is found that the conformation at the minimum energy point is gauche3.
Activation Energy, Ea
In order to calculate the activation energy the structure of both chair and boat transition states were reoptimised using DFT/B3LYPT/6-31G(d). The results are shown as follows.
Chair TS, DFT/B3LYPT/6-31G(d)
Item Value Threshold Converged? Maximum Force 0.000027 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000107 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy=-5.283774D-09 Optimization completed. -- Stationary point found.

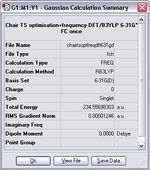
Boat TS, DFT/B3LYPT/6-31G(d)
Item Value Threshold Converged? Maximum Force 0.000017 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000729 0.001800 YES RMS Displacement 0.000175 0.001200 YES Predicted change in Energy=-3.623702D-08 Optimization completed. -- Stationary point found.

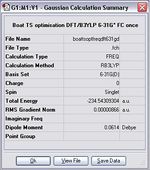
The following table shows the comparison between the energies, geometries from HF/3-21 and that from DFT/B3LYPT/6-31G(d).
| Energies/Geometries | Chair TS (HF/3-21G) | Chair TS (DFT/B3LYPT/6-31G(d) ) | Boat TS (HF/3-21G) | Boat TS (DFT/B3LYPT/6-31G(d) ) |
|---|---|---|---|---|
| Electronic energy/ Hartree | -231.61932 | -234.55698 | -231.60280 | -234.54309 |
| Sum of electronic and zero-point energies at 298.15 K/ Hartree | -231.46670 | -234.41493 | -231.45092 | -234.40234 |
| Sum of electronic and thermal energies at 298.15 K/ Hartree | -231.46134 | -234.40901 | -231.44529 | -234.39601 |
| Sum of electronic and thermal enthalpies at 298.15 K/ Hartree | -231.46040 | -234.40806 | -231.44435 | -234.39506 |
| Sum of electronic and thermal free energies at 298.15 K/ Hartree | -231.49521 | -234.44381 | -231.47967 | -234.43175 |
| Distance between fragments/ Å | 2.0 | 2.0 | 2.1 | 2.2 |
| C-C-C bond angle/ ° | 120.5 | 120.0 | 121.7 | 122.3 |
| Frequency of Vibration/ cm-1 | 818 | 566 | 840 | 531 |
The activation energies, Ea, for both the transition states at 298.15 K were calculated by computing the difference between sum of the electronic and zero-point energies of Anti A and sum of the electronic and zero-point energies of cahir/boat TS from the DFT/B3LYPT/6-31G(d) method. The values from the calculation are tabulated in the following table.
| TS | Ea at 298.15 K/ Hartree | Ea at 298.15 K/ kcal/mol | Literature values | Experimental values |
|---|---|---|---|---|
| Chair TS | 0.05208 | 32.68 | 33.17 | 33.5 ± 0.5 |
| Boat TS | 0.06467 | 40.58 | 41.32 | 44.7 ± 2.0 |
The pathway via the boat transition state possesses a higher activation energy than that via chair transition state. This is, the boat transition state is less stable compared the the chair transition state. In kinetic point of view, the reaction route via the chair transition state is more favourable than the one via the boat transition state. The activation energies obtained from the computation using DFT/B3LYPT/6-31G(d) method are very close to both literature and experimental results.
Diels Alder Reactions
The Diels Alder reaction is classified as a pericyclic reaction. The π orbitals of the dieneophile interact with the π orbitals of the diene to form new σ bonds. In order to judge whether the reaction will take place or not, the number of π electrons involved in the reaction must be counted. That is, the reactions will undergo via concerted mechanism or not depends on the number of π electrons involved in the reaction. The Woodward-Hoffmann rule proposed by Woodward and Hoffmann in 1965 states that thermal electrocyclic reaction involving 4n, where n is an integer, electrons is allowed if the reaction undergoes via conrotatory fashion. While the reaction involving 4n+2 electrons is allowed if it proceeds by disrotatory path. [4] In this study two Diels-Alder reactions; namely the reaction of ethene and cis-butadiene and the reaction of cyclohexadiene and maleic anhydride, will be investigated in terms of their transition states and molecular orbitals (MOs).
The reaction between 1,3-butadiene and ethene
This Diels Alder reaction involves 6 π-electrons. Therefore, according to the Woodward-Hoffmann rules this reaction is allowed to proceed via disrotatory path if the reaction is in thermal conditions.
In the reaction the cis-butadiene contributes 4 π-electrons to the system and the ethene contributes 2 π-electrons to the system. As a result, this reaction is called [4+2] cycloaddition reaction.
Optimisation
Cis-butadiene
Item Value Threshold Converged? Maximum Force 0.000025 0.000450 YES RMS Force 0.000011 0.000300 YES Maximum Displacement 0.000318 0.001800 YES RMS Displacement 0.000128 0.001200 YES Predicted change in Energy=-8.152975D-09 Optimization completed. -- Stationary point found.

The structure of cis-butadiene was successfully optimised to the minimum energy (0.04880 a.u.). The geometry of the optimised cis-butadiene is summarised in the following table.
| Structure | C-C bond lenght/Å | C=C bond lenght/Å | C=C-C bond angle/° | symmetry |
|---|---|---|---|---|
| cis-butadiene | 1.45 | 1.34 | 123.7 | C2v |
Transition state analysis
TS
Item Value Threshold Converged? Maximum Force 0.000002 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000008 0.001800 YES RMS Displacement 0.000002 0.001200 YES Predicted change in Energy=-1.519152D-11 Optimization completed. -- Stationary point found.

The transition state vibrates at -1031 cm-1.
The vibration at such a frequency shown above indicates the bond formation/breaking between the butadiene and ethene.
The geometry of the transition state is summarised in the following table.
| Structure | C-C bond lenght/Å | C=C bond lenght (diene fragment)/Å | C=C-C bond angle/° | C=C bond lenght (dienophile fragment/Å | Distances between two fragments/Å |
|---|---|---|---|---|---|
| Transition state | 1.40 | 1.45 | 92.5 | 1.38 | 2.13 |
Molecular orbital analysis
In order to explain the Woodward-Hoffmann rules, the frontier orbitals of diene and the transition state must be investigated.
Cis-butadiene
| HOMO of cis-butadiene at -0.34550 a.u. | LUMO of cis-butadiene at 0.01876 a.u. |
TS
| HOMO of cis-butadiene at -0.30067 a.u. | LUMO of cis-butadiene at 0.01287 a.u. |
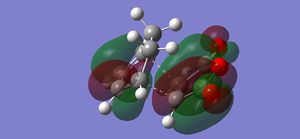
The HOMO of the transition state clearly shows the bonding orbitals between the diene and the dienophile.
The reaction 1,3-cyclohexadiene and maleic anhydride
Transition state analysis
Endo-transition state
Item Value Threshold Converged? Maximum Force 0.000033 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.001591 0.001800 YES RMS Displacement 0.000281 0.001200 YES Predicted change in Energy=-5.989757D-08 Optimization completed. -- Stationary point found.

The transition state vibrates at -806 cm-1.
| HOMO of cis-butadiene at -0.34505 a.u. | LUMO of cis-butadiene at -0.03570 a.u. |
Exo-transition state
Item Value Threshold Converged? Maximum Force 0.000105 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.001235 0.001800 YES RMS Displacement 0.000273 0.001200 YES Predicted change in Energy=-1.023489D-07 Optimization completed. -- Stationary point found.
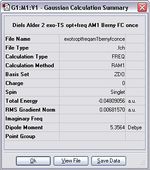
The transition state vibrates at -821 cm-1.

| HOMO of cis-butadiene at -0.34153 a.u. | LUMO of cis-butadiene at -0.03596 a.u. |