Rep:Mod:AM8709mod1
Molecular calculations using molecular mechanics
The hydrogenation of cyclopentadiene dimer
The dimerisation of cyclopentadiene can undergo when two molecules of cyclopentadiene 1 react with each other via a pericyclic reaction—Diels-Alder reaction. There are two isomers can possibly form from such a reaction: endo 2 and exo 3 products, Scheme 1.
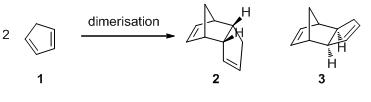
Endo rule proposed by Alder and Stein in the 1930s states that endo products from Diels-Alder reaction are usually obtained in higher yield than exo products (Alder, Stein 1937). In 1965, Woodward and Hoffmann suggested that such a rule may be explained by the frontier orbitals of diene and dienophile. (JACS Woodward and Hoffmann 1965). The transition state of the endo product Fig. 1 shows the secondary orbital interaction; such an interaction lowers the energy of the transtion state for the endo addition relative to that for the exo addition. As a result, the reaction can undergo via the endo addition path more quickly than via the exo addition path. That is, in Diels-Alder reaction, which normally is kinetically controlled, endo products are usually gained as major products. However, once endo isomers are heated, they tend to convert to exo product (JACS Mueller 1961). That is, in thermodynamically controlled conditions exo products are more likely to form as major products, not exo products. Hence, exo isomers are said thermodynamic products while endo isomers are kinetic products.

In this study, Allinger MM2 Molecular Mechanics force field was used to optimise the structures of the two products 1 and 2. The results from the optimisation are tabulated in Table 1. Unsurprisingly, the data from the calculations show that the exo product has lower total energy than that for the endo product as expected since the exo product as mentioned earlier is a thermodynamic product, more stable than the endo product. Unfortunately, the calculations which can illustrate that the energies of the transition states of endo and exo additions cannot be done by the Molecular Mechanics force field. Because the molecular wavefunctions of the species at the transition states have to be taken into account, which is beyond the capability of such a method.
| Results | ''Exo'' product | ''Endo'' product | Energy difference/ kcal mol-1 | % contribute to the difference in total energy |
|---|---|---|---|---|
| Stretch/ kcal mol-1 | 1.2861 | 1.2507 | 0.0354 | 1.5% |
| Bend/ kcal mol-1 | 20.6031 | 20.8656 | 0.2625 | 11.1% |
| Stretch-Bend/ kcal mol-1 | -0.8418 | -0.8304 | (-)0.0114 | 0.5% |
| Torsion/ kcal mol-1 | 7.6545 | 9.5035 | 1.8490 | 78.1% |
| Non-1,4 Van der Waals/ kcal mol-1 | -1.4326 | -1.5162 | (-)0.0836 | 3.5% |
| 1,4 Van der Waals/ kcal mol-1 | 4.2351 | 4.2936 | 0.0585 | 2.5% |
| Dipole/Dipole/ kcal mol-1 | 0.3773 | 0.4452 | 0.0679 | 2.8% |
| Total energy/ kcal mol-1 | 31.8817 | 34.0120 | 2.1303 | - |
Let consider the main reasons which cause the less stability in the endo product compared to that in the exo product. From Table 1 we will see that torsion is the key factor that leads to the difference in total energy of the two products (78.1%). This is due to the fact that the structure of the endo product is bended on itself causing high torsion, which leads to high steric hindrance. Unlike the endo product, one of the ring in the exo product points away from the main ring creating low torsion. Hence, low steric hindrance.
Let move to the second part of the study: the hydrogenation of the endo dimer, Scheme 2. The hydrogenation in short period of time can produce one of the dihydro derivatives, either 3 or 4.

The same method of calculations was applied to optimise the dihydro derivatives 3 and 4. The data from the optimisation are summarised in Table 2.
| Results | Dihydro derivative 3 | Dihydro derivative 4 | Energy difference/ kcal mol-1 | % contribute to the difference in total energy |
|---|---|---|---|---|
| Stretch/ kcal mol-1 | 1.2659 | 1.0936 | 0.1723 | 2.0% |
| Bend/ kcal mol-1 | 19.8063 | 14.5171 | 5.2892 | 60.9% |
| Stretch-Bend/ kcal mol-1 | -0.8276 | -0.5456 | (-)0.2820 | 3.2% |
| Torsion/ kcal mol-1 | 10.8698 | 12.5095 | 1.6397 | 18.9% |
| Non-1,4 Van der Waals/ kcal mol-1 | -1.2207 | -1.0642 | (-)0.1565 | 1.8% |
| 1,4 Van der Waals/ kcal mol-1 | 5.6394 | 4.5117 | 1.1277 | 13.0% |
| Dipole/Dipole/ kcal mol-1 | 0.1621 | 0.1407 | 0.0214 | 0.2% |
| Total energy/ kcal mol-1 | 35.6953 | 31.1627 | 4.5326 | - |
According to Table 2, it is obviously to see that the product 4 has lower total energy than that for the product 3, i.e., the product 4 is thermodynamically more stable than the product 3. This is mostly due to bend (60.9%) and torsion (18.9%) or in the other word ring strain and steric hindrance effects. The alkene within the six-membered ring of the product 3 introduces the higher ring strain relative to the one within the five-membered ring of the product 4. This is because the bond angles at an sp2carbon atom are ideally 120°. Yet the bond angles at the sp2 carbon atoms along the carbon backbone in the product 3 are 107.8°, meanwhile the ones in the product 4 are 113.1°. As a result, the product 4 has lower ring strain than the product 3 as the bond angles within the product 4 are closer to the ideal one. In agreement with the calculations, the result from the experiment confirms that the principal product obtained from the hydrogenation is the product 4 (Guozhu Liu 2005 Ind Eng Chem Res). Therefore, it can be concluded that the hydrogenation of the endo dimer of cyclopentadiene is a thermodynamically controlled reaction.
To sum up the dimerisation of cyclopentadiene is a kinetically controlled process, so the kinetic product, the endo dimer, is obtained. However, the calculations showing that the transition state of the endo dimer has lower energy than that for the exo dimer cannot be done using the Molecular Mechanics approach as the molecular wavefunctions must be taken into account. Unlike the dimerisation, the hydrogenation of the endo dimer is a thermodynamically controlled reaction. As a result, the thermodynamic product, 4, is yielded. However, once the hydrogenation process is prolonged, the fully hydrogenated product, tetrahydro derivative 5, would be obtained.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol

Taxol is an important drug for the treatment of ovarian cancer. In the total synthesis of Taxol, one of the key intermediate shown in Fig. 2 was investigated. There are two atropisomers of such an intermediate; one with the carbonyl group points above the plane 9, the other with the carbonyl points below the plane 10. Atropisomers are stereroisomers caused by hindered rotation about sigma bonds where the steric barrier for the rotation is high enough to allow for the isolation of the conformers (Bringmann G, Mortimer AJP 2005 Angew Chemie Int). These two atropisomers slowly inter-convert to each other; which form is predominant depends on the conditions(Elmore and Paquette 1991 tetrahedron letters). In this study MM2 and MMFF94 force fields were used to optimise the structures of the two atropisomers. For each atropisomer, there are two chair conformations A and B with different energy. Let say A possesses lower energy than B. The data from the computations are tabulated in Table 3.
| Results | 9A CO up | 9B CO up | 10A CO down | 10B CO down |
|---|---|---|---|---|
| Stretch/ kcal mol-1 (MM2) | 2.7846 | 3.3214 | 2.6211 | 3.3152 |
| Bend/ kcal mol-1 (MM2) | 16.5395 | 20.5006 | 11.3419 | 16.8095 |
| Stretch-Bend/ kcal mol-1 (MM2) | 0.4302 | 0.4990 | 0.3437 | 0.4357 |
| Torsion/ kcal mol-1 (MM2) | 18.2533 | 22.0083 | 19.6644 | 20.1197 |
| Non-1,4 Van der Waals/ kcal mol-1 (MM2) | -1.5521 | -1.0727 | -2.1582 | -0.1277 |
| 1,4 Van der Waals/ kcal mol-1 (MM2) | 13.1090 | 14.9620 | 12.8722 | 13.7746 |
| Dipole/Dipole/ kcal mol-1 (MM2) | -1.7249 | -1.8328 | -2.0021 | -1.7844 |
| Total energy/ kcal mol-1 (MM2) | 47.8395 | 58.3859 | 42.6829 | 52.5427 |
| Total energy/ kcal mol-1 (MMFF94) | 70.5606 | 82.808 | 60.5663 | 74.7867 |
We cannot compare the magnitudes of the total energy obtained from different force fields. However, we can do compare the values obtained from the same force field but different strutures. As a result, according to Table 3 the values of total energy gained from MM2 method of 9A, 9B, 10A, and 10B can be compared. First let consider 9A and 10A, both are the lowest energy conformers of the two atropisomers. 10A possesses lower total energy than 9A. Likewise for 9B and 10B, 10B has lower total energy than 9B. Therefore, we can conclude that the atropisomer with the carbonyl pointing below the ring 9 is thermodynamically more stable than 10. This might be because of the dihedral angle between the carbonyl group and the hydrogen on the adjacent carbon on the cyclohexyl ring. The conformers 9A and 10A have larger dihedral angles compared to the the conformers 9B and 10B. As a result, the former pairs have less steric hindrance than the latter. The same idea can be applied for the conformers 9A and 10A, the dihedral angle for 10A is 167° while for 9A is 56°. Hence, 10A has less steric hindrance effect and more stable than 9A, i.e. 10A is the stable conformer for one atropisomer. However, 9A is still the stable form for the other atropisomer.
The reason for the alkene within this intermediate is inert can be explained using the concept of hyperstable olefin (David Hammon Guy JOC 1992). This type of alkene is classified as a bridge-head alkene, and the system, for example, polycyclic rings are stabilised by the hyperstability of the alkene. Such a stability. Once the unsaturated polycyclic system is compared to its parent saturated ring, the unsaturated system is amazingly more stable than the saturated hydrocarbon system (McEwen Paul JACS 1986).
Molecular calculations using semi-empirical molecular orbital theory
Regioselective Addition of Dichlorocarbene

In this study the regioselectivity of the reaction between dichlorocabene species and compound 12 was investigated. Since there are two alkenes within the compound. To decide which alkene is more reactive to the carbene, the frontier orbitals of the compound 12 have to be simulated. In order to investigate the molecular orbitals of 12 the molecular wavefunction of such a compound has to be taken into account. As a result the semi-empirical method was used to calculate the molecular orbital of the molecule. However, the structure was optimised using MM2 force field in order to reduce time taken in the calculation in the the semi-empirical approach. Because MM2 method of optimisation can provide a fair structure as a good beginning for the semi-empirical calculation. PM6 semi-empirical was used to generate the molecular orbitals in the molecule. The pictures of HOMO-1, HOMO, LUMO and LUMO+1 are shown below.




By inspection the shapes of the molecular orbital obtained from the semi-empirical calculations, we can say that they are reasonable results. Because the structure of compound 12 has a plane of symmetry aligned along the Cl-C-H, the simulations obtained from the calculation also remain the same plane of symmetry, i.e. the symmetry of the molecule is conserved. From the molecular orbital of HOMO, "the highest density" (biggest lobes) of electrons are located on the alkene on the same side of the chlorine atom. This implies that such an alkene is the most reactive functional groups within this molecule towards electrophilic attack, i.e. good nucleophile. In addition the molecular orbital also indicates that the two alkene of this molecule are obviously different in terms of reactivity. By orbital analysis, dichlorocarbene is more likely to react with the alkene on the same side of the chlorine atom. The IR spectra of compound 12 and compound 13 were generated from DFT B3LYP method with 6-31G(d,p)basis sets. The spectra are shown below.


There are two main peaks: C=C stretches and C-Cl stretches. First let consider the IR spectrum of compound 12.The C-Cl stretches of compound 12 are at 770.86 cm-1 and the C=C stretches are at 1737.14 cm-1 (the one on the same side of the hydrogen atom) and at 1757.37 cm-1 (the one on the same side of the chlorine atom) For compound 13, The C-Cl stretches signal is at 780.06 cm-1 and the signal of C=C stretches is at 1753.76 cm-1
Both compounds give the similar spectra, however the major difference is there are two peaks for C=C stretches in the IR spectrum of compound 12 while the spectrum of compound 13 shows one peak of the same stretches. This confirms that the semi-empirical and DFT calculations can differentiate these two types of alkene.
Monosaccharide chemistry glycosidation

In this study the effect of neighbouring group in sugar molecule was investigated, as shown in Fig. 10.
The total energy of each isomer of the sugar molecules was calculated from Molecular Mechanics and semi-empirical PM6 methods. The results from the calculation are summarised in Table 4.
In this study the results obtained from semi-empirical approach is better, because molecular orbitals play a key role in anomeric effect. That is, Molecular Mechanics cannot take the wavefunction of the molecule into account as it uses a pure concept of classical mechanics. On the other hand PM6 method uses both quantum and classical mechanics in calculations. According to Table 4, the isomer with acetyl group above the ring (B and B') is more stable than the other isomer, acetyl group below the ring (A and A'). Consequently, the α-anomer is more likely to form.
| Results | A | A' | B | B' |
|---|---|---|---|---|
| Total energy/ kcal mol-1 (MM2) | 29.6614 | 19.3626 | 20.4391 | 22.2325 |
| Total energy/ eV (PM6) | -3346.96827 | -3347.42068 | -3347.44285 | -3346.87885 |
Since there are four possible structures for the starting material (A, A', B and B'), they can give also four possible intermediates (C, C', D and D').
Mini project: Stereoselective dissolving metal reductions
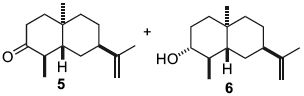
Reduction reactions play an important roles in organic chemistry. In this mini project we will examine the stereoselectivity of Birch reduction, dissolving lithium metal in liquid ammonia. The model reaction for the study comes from the paper by Castellanos et. al. in 2006. The paper shows that the ketone 9 was reduced using Birch reduction to alcohol 6 stereospecifically. The proposed mechanism is shown in Scheme. The 13C NMR chemical shifts and the optical rotation of the product were predicted using DFT GIAO approach. The comparison of the results obtained from the computation and the literature values are tabluated in Table 5.
| 13C NMR chemical shifts from DFT GIAO approach | 13C NMR chemical shifts from literature |
|---|---|
| 147.1087 | 147.11 |
| 112.9907 | 110.67 |
| 76.5320 | 76.86 |
| 45.1240 | 43.22 |
| 43.2265 | 39.93 |
| 42.1477 | 39.19 |
| 41.5272 | 38.88 |
| 40.5994 | 37.10 |
| 37.9075 | 33.78 |
| 35.5062 | 30.92 |
| 29.7774 | 26.05 |
| 27.4492 | 23.08 |
| 26.9743 | 22.82 |
| 20.0051 | 16.68 |
| 18.9526 | 14.84 |
For the 13C NMR chemical shifts, the predicted chemical shifts are close to the literature values (Tetrahedron 63, 7, 2007). On the other hand, the optical rotation (-28°) is a lot further away from the experimental result (-11.6°).
