Rep:Mod:AM7412in
Inorganic Computational Study
Introduction
Several experimental properties for inorganic systems, such as vibrational studies, bonding contributions and molecular orbital analysis can be understood by conducting computational calculations on these systems to investigate more complex modes of bonding, such as multi-centre bonding in dimer structures. This project uses the Gaussian program to geometrically optimise chemical structures, conduct frequency analysis, predict charge distributions and visualise molecular orbitals to observe how they differ from those predicted by the linear combination of atomic orbitals (LCAO) method.
A molecule is "optimised" by studying its potential energy surface (PES) in an attempt to find a local minimum, with the first derivative of the potential energy surface equalling zero, corresponding to an "optimised" conformation. Initially points of zero gradient are located, these are then subsequently analysed using a second derivative of energy to determine the overall curvature of said point on the PES. The gradient of a second derivative can then be identified as an unwanted point of inflection, maxima (transition state) or the desired energy minimum. A positive value of the second derivative corresponds to the desired energy minimum which in turn corresponds to an optimised low energy conformation of a specific structure.
Optimisation of EX3 Structures
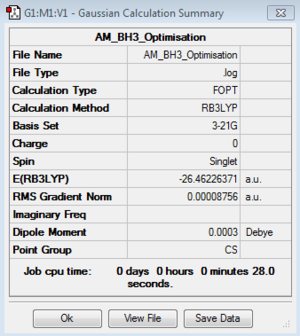
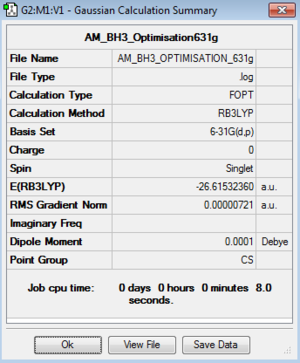
Optimisation of BH3:B3LYP/3-21G level
Optimisation log file here
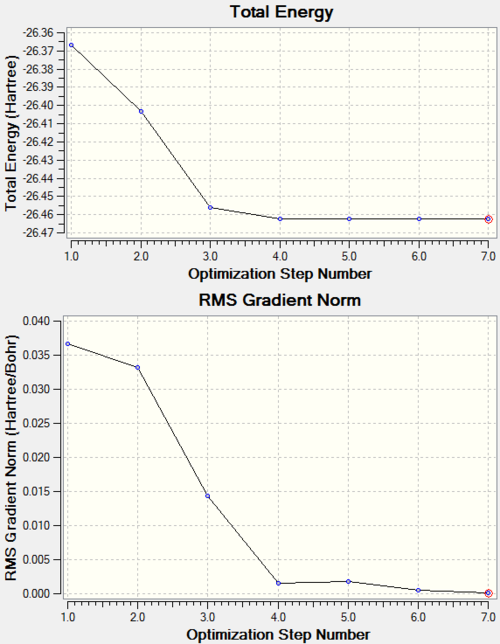
RMS Curves
The RMS curves show that it took 7 steps identify a minimum on the potential energy surface and locate an optimised geometry using the 3-21G basis set.
Optimisation of BH3:B3LYP/6-31G(d,p) level
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|
|
Item Value Threshold Converged? Maximum Force 0.000012 0.000450 YES RMS Force 0.000008 0.000300 YES Maximum Displacement 0.000064 0.001800 YES RMS Displacement 0.000039 0.001200 YES |
|
Optimisation of GaBr3:B3LYP/LANL2DZ level
Optimisation file: DOI:10042/195215
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|
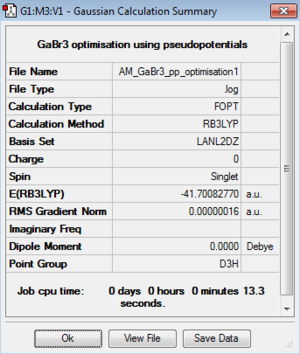
|
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000003 0.001800 YES RMS Displacement 0.000002 0.001200 YES |
|
Optimisation of BBr3:B3LYP/LANL2DZ level
Optimisation log file here
Optimisation file: DOI:10042/195227
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|
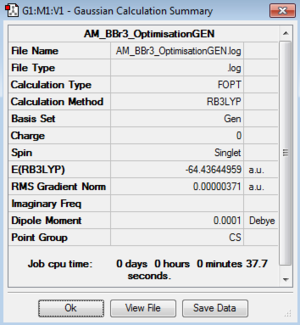
|
Item Value Threshold Converged? Maximum Force 0.000008 0.000450 YES RMS Force 0.000005 0.000300 YES Maximum Displacement 0.000035 0.001800 YES RMS Displacement 0.000023 0.001200 YES |
|
Geometry Comparison
| BH3 | BBr3 | GaBr3 | |
|---|---|---|---|
| r(E-X) Å | 1.19 | 1.93 | 2.35 |
| θ(X-E-X) degrees(º) | 120.0 | 120.0 | 120.0 |
As can be seen from the table above, upon changing the ligand from a hydrogen atom to a bromine atom an increase of ~0.74 Å (62%) is observed. This effect arises due to the larger nature of bromine's atomic radius, which is much larger than a hydrogen atoms' radius. The electron configuration of bromine is [Ar]3d104s24p5, with the presence of lone pairs which can be donated into the vacant p-orbital of boron, whilst the configuration of hydrogen is 1s1. The interaction of the lone pair in bromine which is found in a p-orbital results in pπ-pπ backbonding, which is absent in BH3. The absence of the backbonding interaction makes BH3 a better lewis acid as the vacant p-orbital is more readily available to accept electron density. The B-H and B-Br bonds are both single bonds and due to the increased electronegativity of the bromine atom the B-Br bond is highly polar making it stronger than the B-H bond in theory. However, it can be argued that the pπ-pπ backbonding is not significantly large because of the mismatch between the 4p orbitals on bromine and the 2p orbital on boron as well as the increase in atomic radii of the ligand taking precedence which results in a lengthening of the B-Br bond, making it weaker than the B-H bond.
The comparison between GaBr3 and BBr3 involves investigating the effect of changing the central atom, whilst keeping the ligands constant. Gallium has a larger atomic radius than boron resulting in the longer bond length observed. However, this increase is not as prominent compared to when the ligand is altered as an increase of only 0.42 Å (22%) is seen. This may be explained due to the fact that both gallium and bromine are in the same period, with the same principle quantum number allowing for a good orbital overlap (4pπ-4pπ), due to similar sized atomic radii leading to the formation of a strengthened and shortened Ga-Br bond. However, as with the previous example this backbonding/overlap effect is not enough to outweigh the difference in the atomic radius between gallium and boron and thus a longer bond length is observed upon an increase in the atomic radius of the central atom.
Defining a bond both qualitatively and quantitatively can be a challenge and is still debatable to date. However, one can assume that the formation of a bond (a physical connection between two atoms) occurs at an equilibrium state (attractive interaction) between the distance of the interacting atoms being significantly larger than their combined Van der Waal's radii and the distance being significantly less than their combined Van der Waal's radii (repulsive interaction). This can be represented diagrammatically by the Lenard-Jones potential. A bond can have many various froms ranging from an ionic bond in LiCl for example to a non-covalent hydrogen bond. Using computational methods such as GaussView, if a calculation results in a bond length with a magnitude above a certain distance the bond does not appear. This distance between the atoms is based on quantitative data which forces GaussView to define what a bond is and what doesn't fall into this category. On the other hand, a more subjective view is that if the electronic interaction between two atoms is sufficient enough to consider the atoms to be part of a single system, in which each atom is dependent on the other, regardless of the distance between them this can be considered a bond. However, above a certain distance this electronic interaction would be very weak and therefore bond distance is a useful tool for measuring the strength of a chemical bond. Bond strength can be further explored and categorised into strong, medium and weak bonds. Covalent bonds (adjacent atoms sharing a pair of electrons) are examples of very strong bonds, a typical carbon-oxygen (C=O) double bond has an energy of around 732kJ mol-1 1. A medium bond strength is observed for O-H bond with an energy of approximately 372kJ mol-1.2 Finally, hydrogen bonds are an example of a weak bond as they are a non-covalent molecular interaction with energies between 4-13kJ mol-1.23
Another way of interpreting bond strength is the length of the bond. In general longer bonds are weaker than shorter bonds due to the substituents at each end repelling causing both a lengthening and a weakening of the bond between them, but also due to the weakening of the force constant of the bond connecting the two atoms/groups of atoms. A further method for studying the strength of a bond is IR spectroscopy which utilities the Harmonic Oscillator model and treats a bond as a spring between two masses. The vibrational frequency of a bond is directly proportional to the force constant (k), which is a second derivative of potential energy against bond distance and is used to determine the curvature of a potential energy surface (PES).4 During all of the calculations above the PES is being explored in order to find a local minimum which corresponds to an optimised geometry. The curvature of the PES can also be used to determine the strength of a bond, a very steep curvature yields a large k value which in turn corresponds to a large vibrational frequency, the larger the vibrational frequency the stronger the bond.4 In contrast a low curvature slope corresponds to a smaller force constant, which would be weaker. In addition to the above a bond can be single, double or triple in nature which all have varying bond strengths, depending on the atoms at either end making the question of "how strong is a bond" even harder to answer. This project aims to present the potential of Gaussian as a computational method for understanding this complex question.
Frequency Analysis
BH3:B3LYP/6-31G(d,p)
Frequency file: here
| summary data | low modes |
|---|---|
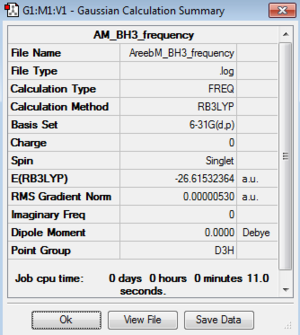
|
Low frequencies --- -14.5183 -14.5142 -10.8197 -0.0009 0.0168 0.3454 Low frequencies --- 1162.9508 1213.1230 1213.1232 |
As the low frequencies are withinn 15 cm-1 a minimum has been found on the potential energy surface and the vibrations/IR spectrum can analytically interpreted.
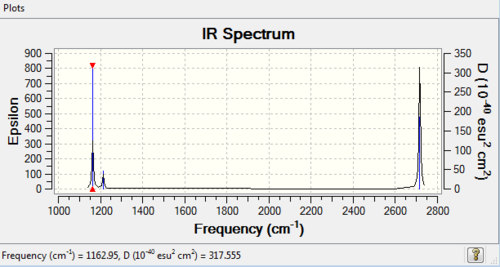
Vibrational spectrum for BH3
| Wavenumber (cm-1) | Intensity | IR active? | Type | Description | Symmetry (D3h) |
|---|---|---|---|---|---|
| 1163 | 93 | yes | bend | BH3 Umbrella Deformation: All 3 hydrogen's perform a wagging motion above and below the plane of the boron central atom | A2 |
| 1213 | 14 | very slight | bend | BH2 Rocking: Two hydrogen's perform a rocking motion whilst the third hydrogen bends with a larger magnitude | E' |
| 1213 | 14 | very slight | bend | BH3 Scissoring Motion: Two hydrogen's perform a scissoring action whilst the third hydrogen and the boron are effectively stationary | E' |
| 2583 | 0 | no | stretch | Symmetric H3 Motion: All 3 hydrogen's are stretching symmetrically away from the stationary boron - no change in the overall dipole moment and thus this mode is IR inactive | A1' |
| 2716 | 126 | yes | stretch | Asymmetric BH Stretch: Two hydrogen's undergo asymmetric stretching whilst the third hydrogen and the boron atom remain stationary | E' |
| 2716 | 126 | yes | stretch | Symmetric BH Stretch: Two hydrogen's stretch symmetrically whilst the third hydrogen stretches asymmetrically with a greater magnitude | E' |
GaBr3:B3LYP/LanL2DZ
Frequency file: here
Frequency file: DOI:10042/195242
| summary data | low modes |
|---|---|
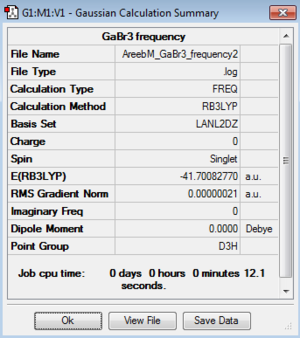
|
Low frequencies --- -1.4877 -0.0015 -0.0002 0.0096 0.6540 0.6540 Low frequencies --- 76.3920 76.3924 99.6767 |
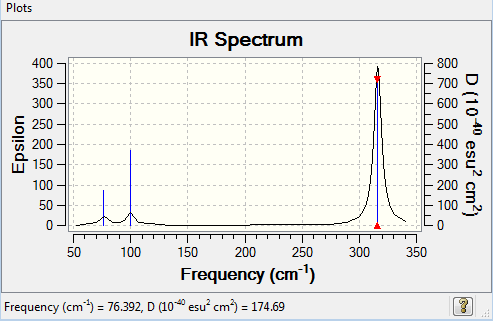
Vibrational spectrum for GaBr3
| Wavenumber (cm-1) | Intensity | IR active? | Type | Description | Symmetry (D3h) |
|---|---|---|---|---|---|
| 76 | 3 | very slight | bend | in-plane bend, scissoring motion | E' |
| 76 | 3 | very slight | bend | in-plane bend, rocking motion | E' |
| 100 | 9 | very slight | bend | out-of-plane bend, wagging motion | A2 |
| 197 | 0 | no | stretch | symmetric motion | A1' |
| 316 | 57 | yes | stretch | asymmetric motion | E' |
| 316 | 57 | yes | stretch | asymmetric motion | E' |
Vibrational Study Comparison
It is clear from the data above that the vibrations in GaBr3 are at much lower frequencies than those for BH3. This is due to the increased mass of both the central atom and the ligand. Modelling the bond as a spring with fixed masses at their ends it follows that the frequency of this vibrating "spring" is inversely proportional to the square root of the reduced mass of the system. The reduced mass of a system μ=m1m2/m1+m2 therefore it can be seen that an increase in the mass of the system will lead to a decrease in the frequency.
For both spectra a similar pattern is observed with three low frequency vibrations, one vibration is missing from the IR spectrum due to a symmetric stretch lacking a change in overall dipole moment and a further set of three high frequency vibrations on both GaBr3 and BH3 which are made up of two degenerate vibrations and thus only three peaks are observed in each spectra, instead of the expected six. The vibrational modes have a slight re-ordering, for BH3 the A2 mode has the lowest frequency, whereas for GaBr3 the A2 mode has the third lowest vibration frequency. Comparing the two A2 modes in each molecule it is evident that there is a large difference in the intensity of these two umbrella motions, BH3 has an intensity of 93 whilst GaBr3 has a much lower value of 9 similarly the frequency of this vibration is 1163 compared to 100 cm-1. Upon comparison of the displacement vectors it can be seen that for GaBr3 it is the central gallium atom which has the largest displacement, however for the same vibrational mode in BH3 it is the hydrogen ligands which have the largest displacement vector which is due to the difference in the reduced mass.
| BH3 Umbrella motion | GaBr3 Umbrella motion |
|---|---|
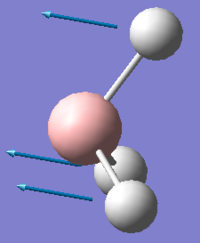
|
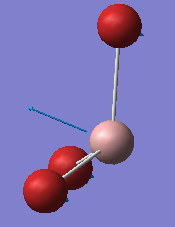
|
In the IR spectra the low frequency modes are typically bends which are generally lower in energy and thus observed at a lower frequency. In contrast stretches are typically higher energy motions and thus occur at higher frequencies.
The same way an optimisation method is undertaken, Gaussian uses analytical derivatives to determine local minima on the potential energy surface (PES), once this is found via an optimisation calculation we carry out a frequency analysis to confirm that the structure is indeed at a potential minimum and not simply at a point where the gradient of the PES is equal to zero. If all the vibrational frequencies are real this confirms that the structure is at a minimum.
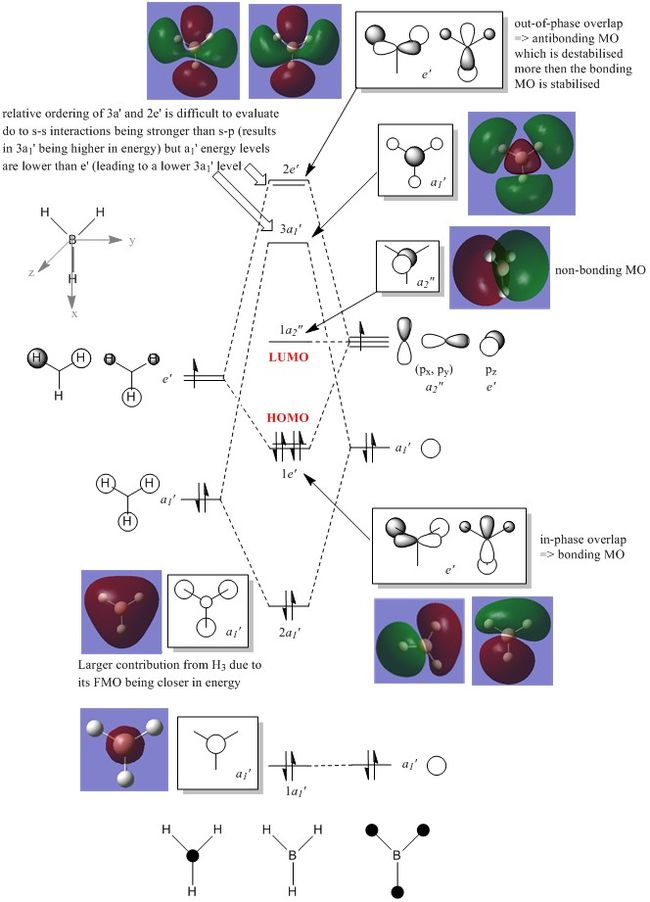
Molecular Orbital Analysis of BH3
MO file: DOI:10042/195350
GaussView was used to compute the molecular orbitals (MO) of BH3 and the MOs generated by the program were used to construct a molecular orbital diagram and analysed to assess the formation of these molecular orbitals and evaluate whether they correlate to the structures predicted by LCAO theory.
Boron and hydrogen are both electropositive elements, with boron being more electropositive than hydrogen due to the availability of an unoccupied p-orbital. As a result, the s-orbitals of both boron and hydrogen will be similar in energy. The combination of 3 hydrogen s-orbitals in phase results in a slight stabilisation and the out-of-phase conformation results in a slight destabilisation as depicted above. Due to the electropositive nature of the boron atom its core s-orbital (1a1') is extremely low in energy and as a result cannot interact with any other orbitals. This large difference in energy (by several magnitudes) between 1a1' and 2a1' is not depicted on the MO diagram above. In BH3 it can be seen that the HOMO is degenerate whilst the LUMO is not and is a non-bonding pz orbital (1a2") on the boron atom. The LUMO is low-lying due to the electropositive nature of boron, making BH3 a susceptible to accepting electrons from nucleophiles with high energy HOMOs. The donation of electrons will not however affect the intrinsic nature (bonding) of BH3 as the LUMO is non-bonding.
Overall, it is evident that the computed MO's are similar to the theorecticals LCAO's. However, as demonstrated by the 1e' and 2e' molecular orbitals, the GaussView computed MO's do not correlate with the shapes predicted by LCAO. The reason behind this is due to GaussView producing orbitals which are extremely diffuse, thus causing their shapes to vary from theory. The molecular orbitals generated are correct in the sense that they show a general bonding (1e') and antibonding character (2e'). One can conclude that it is not necessarily ideal to follow qualititive descriptions of molecular orbitals. Computational techniques will generate different molecular orbitals depending on the geometric position of the structure as well as its optimised energetic state. LCAO theory gives a good visual approximation to the MO's but cannot be used to predict the shape and energy levels of more complex orbitals which arise from mixing
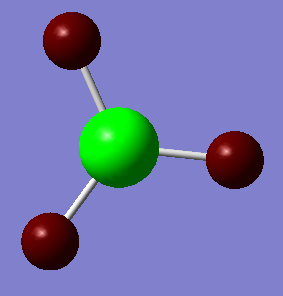
NBO Analysis of BH3
| Charge distribution Summary | Charge distribution colours | Charge distribution values |
|---|---|---|
|
|
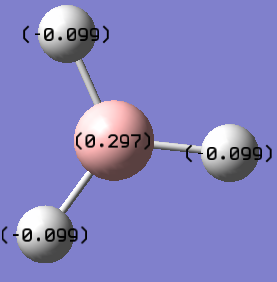
B: 0.297, H: -0.099 |
Association Energy Calculation of Ammonia-borane
Analysis of NH3
Optimisation:B3LYP/6-31G level
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|
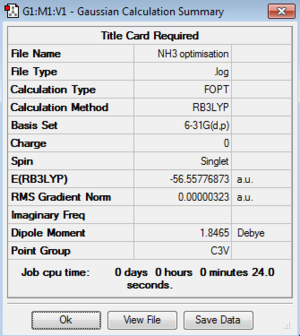
|
Item Value Threshold Converged? Maximum Force 0.000006 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000012 0.001800 YES RMS Displacement 0.000008 0.001200 YES |
|
Frequency:B3LYP/6-31G level
Frequency file: here
| summary data | low modes |
|---|---|
|
Low frequencies --- -0.0139 -0.0025 -0.0010 7.0781 8.0927 8.0932 Low frequencies --- 1089.3840 1693.9368 1693.9368 |
Vibrational spectrum for NH3
| Wavenumber (cm-1) | Intensity | IR active? | Type | Description |
|---|---|---|---|---|
| 1089 | 145 | yes | bend | out-of-plane bend, wagging motion |
| 1694 | 14 | yes | bend | in-plane bend, rocking motion |
| 1694 | 14 | yes | bend | in-plane bend, scissoring motion |
| 3461 | 1 | no | stretch | symmetric motion |
| 3590 | 0 | no | stretch | asymmetric motion |
| 3590 | 0 | no | stretch | asymmetric motion |
NBO Analysis
MO file: DOI:10042/195312
| Charge distribution Summary | Charge distribution colours | Charge distribution values |
|---|---|---|
|
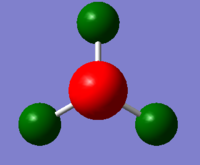
|
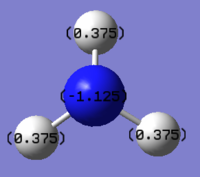
C: -1.125, H: 0.375 |
Analysis of NH3BH3
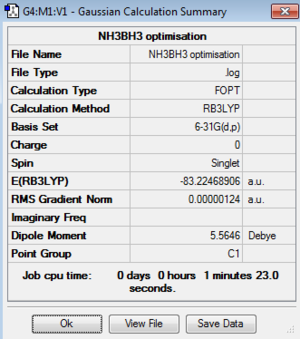
Optimisation of Ammonia-borane:B3LYP/3-21G level
Optimisation log file here
| summary data | convergence | Jmol | |||
|---|---|---|---|---|---|
|
Item Value Threshold Converged? Maximum Force 0.000002 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000026 0.001800 YES RMS Displacement 0.000009 0.001200 YES |
|
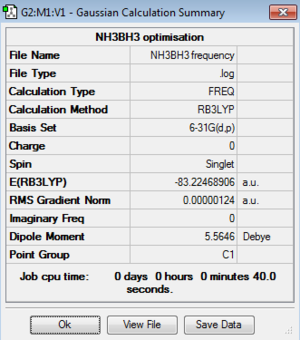
Frequency of Ammonia-borane:B3LYP/3-21G level
Frequency file: here
| summary data | low modes |
|---|---|
|
Low frequencies --- -5.2968 -1.7341 -0.0010 0.0006 0.0012 1.7651 Low frequencies --- 263.3756 632.9648 638.4246 |
Vibrational Spectrum of Ammonia-borane
| Wavenumber (cm-1) | Intensity | IR active? | Degeneracy | Description |
|---|---|---|---|---|
| 263 | 0 | no | 1 | bend |
| 632 | 14 | yes | 1 | B-N stretch |
| 638 | 4 | very slight | 2 | bend |
| 1069 | 41 | yes | 2 | bend |
| 1196 | 109 | yes | 1 | umbrella bend of the BH3 fragment |
| 1204 | 3 | very slight | 2 | in-plane bends of the BH3 fragment |
| 1329 | 114 | yes | 1 | umbrella bend of the NH3 fragment |
| 1676 | 28 | yes | 2 | in-plane bends of the NH3 fragment |
| 2472 | 67 | yes | 1 | symmetric stretch of the BH3 fragment |
| 2532 | 231 | yes | 2 | asymmetric stretch of the BH3 fragment |
| 3464 | 3 | very slight | 1 | symmetric stretch of the NH3 fragment |
| 3581 | 28 | yes | 2 | asymmetric stretch of the NH3 fragment |
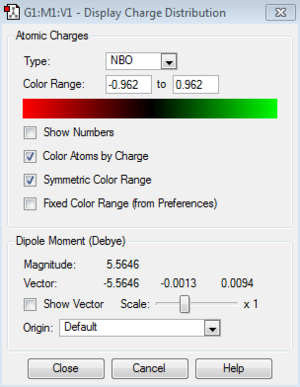
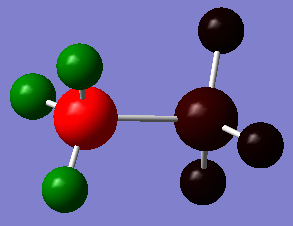
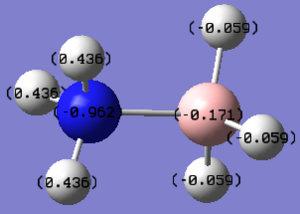
Charge Distribution (NBO) Analysis
MO file: DOI:10042/195313
| Charge distribution Summary | Charge distribution colours | Charge distribution values |
|---|---|---|
|
|
|
Charge Distribution Comparison
| BH3 | NH3 | NH3BH3 |
|---|---|---|
| B-H (-0.099)
B (0.297) |
N-H (0.375)
N (-1.125) |
B-H(-0.059)
B (-0.171) N-H (0.436) N (-0.962) |
From the NBO analysis conducted above it can be seen that due to the electropositive nature of boron, the hydrogen atom is hydridic whereas the electronegative nature of nitrogen in NH3 makes the proton protic. As a result, the ammonia-borane complex produced in the coordination of ammonia with borane has a hydridic and protic site which can result in H2 formation. This mechanism is becoming of increasing importance in industry due to the implications H2 can have on the alternative fuel sector. Upon coordination it is also evident that the protons in BH3 become less basic, whilst the protons in NH3 become more acidic due to the donation of the nitrogen lone pair into the vacant p-orbital of boron.
Enthalpic Analysis of Ammonia-borane formation
| Molecule | Energy (a.u.) | Energy (kJmol-1) |
|---|---|---|
| BH3
NH3 NH3BH3 |
-26.61532360
-56.55776873 -83.22468906 |
-69878.53
-148492.42 -218506.42 |
- where 1 a.u. is 2625.50 kJmol-1
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
ΔE=-83.22468906-[-56.55776873-26.61532360]
ΔE=-0.05159673 a.u.
Therefore, the B-N bond formation energy is -135.48 kJmol-1 and as the dissociation energy is the inverse of bond formation, the enthalpy associated with this process has the same magnitude but opposite sign giving a value of 135.48 kJmol-1, making it an endothermic process as it should be. Therefore, with respect to other known bond energies, this bond can be categorized are relatively weak.
Mini Project - Aromaticity
Introduction
This project was conducted to analyse the structures and molecular orbitals of four isoelectronic aromatic compounds: benzene, boratabenzene, pyridinium and borazine. Along with molecular orbital analysis, the charge distribution across all four molecules, the effect of heteroatom substitution into the ring and analysis of reactivity were all investigated.
All calculations were performed using the DFT/B3LYP method and 6-31G(d,p) basis set, with all molecules being assigned a particular point group which were then subsequently optimised and confirmed through frequency analysis within the standard limits.
Optimisation of Aromatic Compounds
Benzene log file: here
Boratabenzene log file: here
Pyridinium log file: here
Borazine log file: here
| Compound | Summary | Convergence | Jmol | |||
|---|---|---|---|---|---|---|
| Benzene (reference) | 
|
Item Value Threshold Converged? Maximum Force 0.000001 0.000015 YES RMS Force 0.000000 0.000010 YES Maximum Displacement 0.000002 0.000060 YES RMS Displacement 0.000001 0.000040 YES |
| |||
| Boratabenzene | 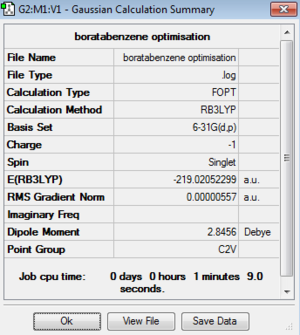
|
Item Value Threshold Converged? Maximum Force 0.000011 0.000015 YES RMS Force 0.000003 0.000010 YES Maximum Displacement 0.000043 0.000060 YES RMS Displacement 0.000012 0.000040 YES |
| |||
| Pyridinium | 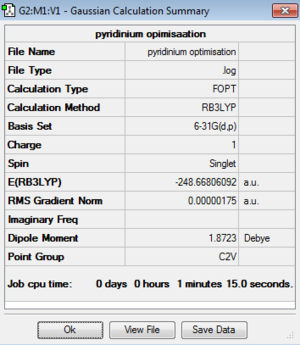
|
Item Value Threshold Converged? Maximum Force 0.000003 0.000015 YES RMS Force 0.000001 0.000010 YES Maximum Displacement 0.000007 0.000060 YES RMS Displacement 0.000002 0.000040 YES |
| |||
| Borazine | 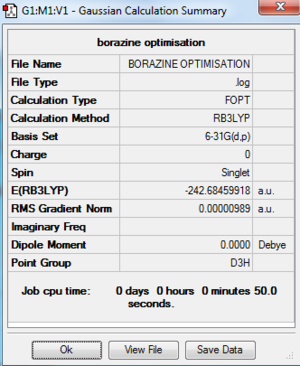
|
Item Value Threshold Converged? Maximum Force 0.000010 0.000015 YES RMS Force 0.000005 0.000010 YES Maximum Displacement 0.000056 0.000060 YES RMS Displacement 0.000018 0.000040 YES |
|
From the table above, all four compounds have been successfully optimised as the RMS gradient has converged.
| Molecule | Energy (a.u.) | Energy (kJmol-1) | Point Group | Bond Lengths |
|---|---|---|---|---|
| Benzene | -232.25820200 | -609793.91 | D6h | C-C: 1.40 Å |
| Boratabenzene | -219.02052299 | -575038.38 | C2v | B-C: 1.51 Å
B-C-C: 1.40 Å C-C: 1.41 Å |
| Pyridinium | -248.66806092 | -652878.00 | C2v | N-C: 1.35 Å
N-C-C: 1.38 Å C-C: 1.40 Å |
| Borazine | -242.68459918 | -637168.41 | D3h | B-N: 1.43 Å |
As the same basis set and calculation method was used to optimise all four structures their energies can be compared. It is clear that the most stable compound out of the four computed is pyridinium. The reason for which is due to electronegative nitrogen atom which lowers the energy of the fragment orbitals making them significantly more stable. The stability of these compounds decreases in the following way: pyridinium > borazine > benzene > boratabenzene. By assessing the point groups of each of the four compounds it can be seen that both benzene and borazine are highly symmetric whereas boratazene and pyridinium are less so with a C2v point group. In benzene the C-C bond is 1.40 Å in length whereas as soon as you add an electropositive atom (boron) the bond length (B-C) increases to 1.51 Å and when you add an electronegative atom (nitrogen) the bond length (N-C) decreases to 1.35 Å. This can be explained due to the polarity of the bond - the more electronegative atoms in a bond are the shorter the bond due to the nuclear pull of the electronegative atom for the bonding pair of electrons and the opposite is true for electropositive atoms.
Frequency Analysis of Aromatic Compounds
Benzene frequency log file: here
Boratabenzene frequency log file: here
Pyridinium frequency log file: here
Borazine frequency log file: here
| Compound | Summary | Low modes |
|---|---|---|
| Benzene (reference) | 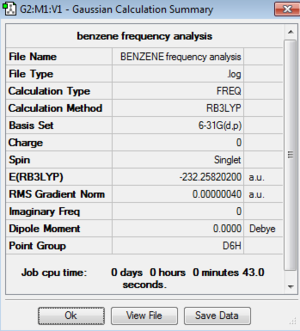
|
Low frequencies --- -10.2549 -5.6651 -5.6651 -0.0055 -0.0055 -0.0003 Low frequencies --- 414.5451 414.5451 621.0429 |
| Boratabenzene | 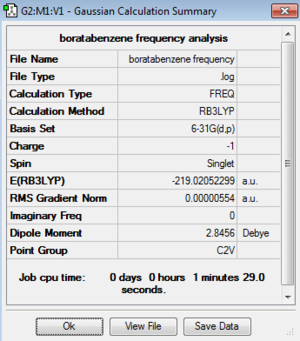
|
Low frequencies --- -7.2728 -0.0011 -0.0009 -0.0007 3.1417 4.5184 Low frequencies --- 371.2968 404.4158 565.0833 |
| Pyridinium | 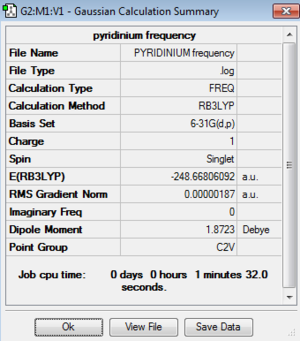
|
Low frequencies --- -9.3821 -2.9805 0.0004 0.0004 0.0009 1.0287 Low frequencies --- 391.9009 404.3423 620.1995 |
| Borazine | 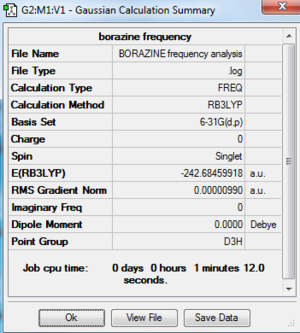
|
Low frequencies --- -4.0533 -4.0509 -3.5449 -0.0037 0.0149 0.0237 Low frequencies --- 289.7385 289.7393 404.5345 |
Since all of the low frequencies are within 15 cm-1 and the frequencies are all real the optimisations have been successful in identifying a true minimum on the PES.
IR Spectrum Analysis
For all four isoelectronic aromatic compounds 30 modes of vibrations were evident upon analysis of the frequency table. However, not all of the modes of vibrations are not IR active, either due to a very small change in dipole moment because of small displacement vectors or due to symmetric stretches which are IR inactive as thet do not result in a net change in dipole moment.
| Compound | Spectra | Description |
|---|---|---|
| Benzene | 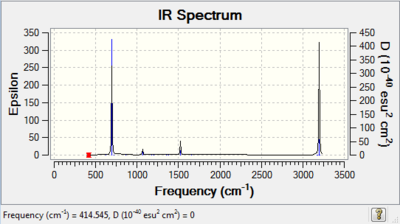
|
Due to the high symmetry of benzene only 4 out of 30 vibrations are IR active. The frequencies of interest are: 1013 cm-1 which corresponds to a ring expansion motion, 695 cm-1 which is an out-of-plane bend and the stretch at 3197 cm-1, corresponding to an asymmetric C-H stretch |
| Boratabenzene | 
|
As boratabenzene is not as symmetrical as benzene therefore more vibrational modes are visible on the IR spectrum. The frequencies for boratabenzene are typically at lower values than that for benzene as a carbon atom has been replaced by a boron atom which is lighter. Of particular interest is the peak at 2447 cm-1 which corresponds to a B-H stretch |
| Pyridinium | 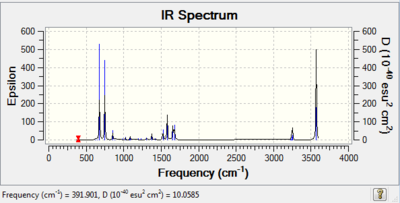
|
The same principles apply to pyridinium as with boratabenzene because pyridinium is also not as symmetrical as benzene therefore more vibrational modes are visible on the IR spectrum. The frequencies for boratabenzene are typically at higher values than that for benzene as a carbon atom has been replaced by a nitrogen atom which is heavier. Of particular interest is the peak at 1300 cm-1 which corresponds to an asymmetric C-N stretch and the peak at 3570 cm-1 which is representative of a N-H stretch. In this instance the N-H stretch is not broad as expected due to H-bonding because the computation is carried out in the gas phase and thus H-bonds are not present - a sharp peak corresponds to a non-H-bonded N-H group |
| Borazine | 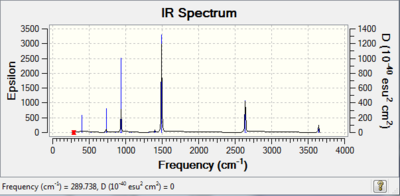
|
Due to the high symmetry of borazine only 9 out of 30 possible vibrational modes are visible in the IR spectrum. More modes are visible in comparison to benzene as borazine is not as symmetric as benzene (benzene - D6h, borazine - D3h). For the same reason as benzene, only a few modes are visible due to the majority not resulting in a change in dipole moment. The peaks of interest are 2640 cm-1 which corresponds to a B-H stretch and 3644 cm-1 which corresponds to an N-H stretch. In both cases the peaks are at higher values to those in boratabenzene and pyridinium |
MO and NBO Analysis of Aromatic Compounds
Benzene MO log file: DOI:10042/195369
Boratabenzene MO log file: DOI:10042/195370
Pyridinium MO log file: DOI:10042/195371
Borazine MO log file: DOI:10042/195372
| Benzene | Boratabenzene | Pyridinium | Borazine |
|---|---|---|---|
|
|
|
|
|
|
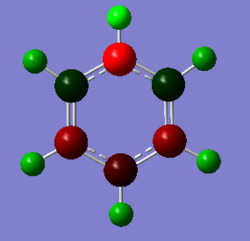
|
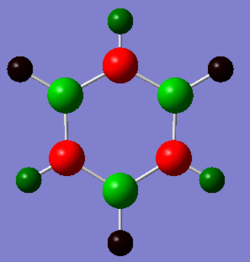
|
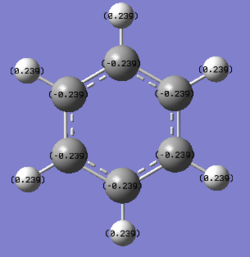
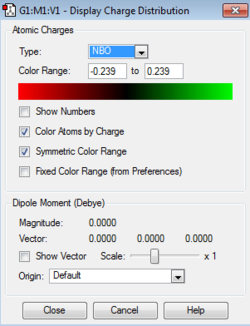
C: -0.239 H: 0.239 |
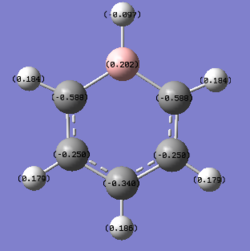
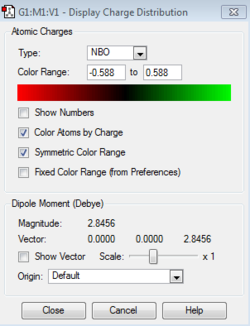
B: 0.202 B-H: -0.097 C: -0.588, -0.250, -0.340 H: 0.184, 0.179, 0.186 |
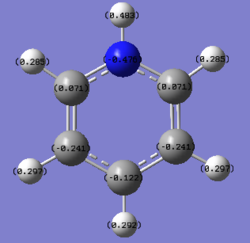
N: -0.476 N-H: 0.483 C: 0.071, -0.241, -0.122 H: 0.285, 0.297, 0.292 |
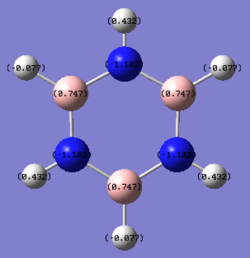
B: 0.747 N: -1.182 B-H: -0.077 N-H: 0.432 |
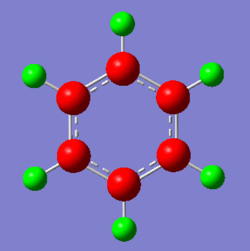
The aromatic compounds under investigation are the isoelectronic species: benzene, boratabenzene, pyridinium and borazine. Benzene is a symmetrical molecule that has D6h symmetry which results in the charges on the atoms of the same type having the same charge with the same magnitude. The summation of the charges across the molecule results in a net zero dipole moment, producing a neutral 6π electron system.
In boratabenzene, the hydrogen atoms have similar charges, whereas the B-H hydrogen is negatively charged and smaller in magnitude. As can be seen the closer the carbon atoms are to the boron atom the more negatively charged they are due to the electropositive nature of boron. The chemical reactivity of boratabenzene can be explained by the charge distribution across the molecule. The boron atom on the molecule has a vacant p-orbital which can be attacked by nucleophiles such as hydridic species resulting in the delocalisation of the negative on the carbon atom. The negative charge can resonate between two different positions, these being the ortho- and para- positions from the boron atom. The spread of the negative charge at these two positions is shown in the charge distribution above where they are more negative than the meta- positions but are also more negative than the carbon atoms in benzene.
In the pyridinium ion, the hydrogen atom bound to the nitrogen has a charge of 0.483. Due to the electronegative nature of the nitrogen atom the carbon atoms close to the nitrogen atom are positive in nature due to the nitrogen atom attracting electron density from the neighbouring atoms. As a result of this effect the atoms are more positive in comparison to benzene. From the charge distribution values it can be seen that the activated positions are the ortho- and para- positions from the nitrogen atom, which can be shown through resonance forms of the compound.
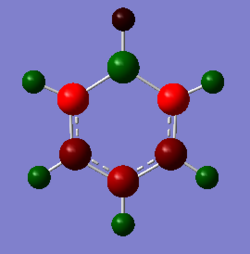
Finally, due to the symmetry in borazine (D3h) all of the boron atoms have the same charge (positively charged), whilst the nitrogen atoms are negatively charged. From this it can be seen that the hydrogen atoms bound to the nitrogen are more positively charged than those bound to the boron atom due to the difference in electronegativity - there is a slight negative charge on the B-H hydrogen atoms even though the molecule is neutral. The charges are more pronounced on these heteroatoms in comparison to their isoelectronic analogues, boratabenzene and pyridinium due to the presence of adjacent electropositive (boron) and electronegative (nitrogen) species.
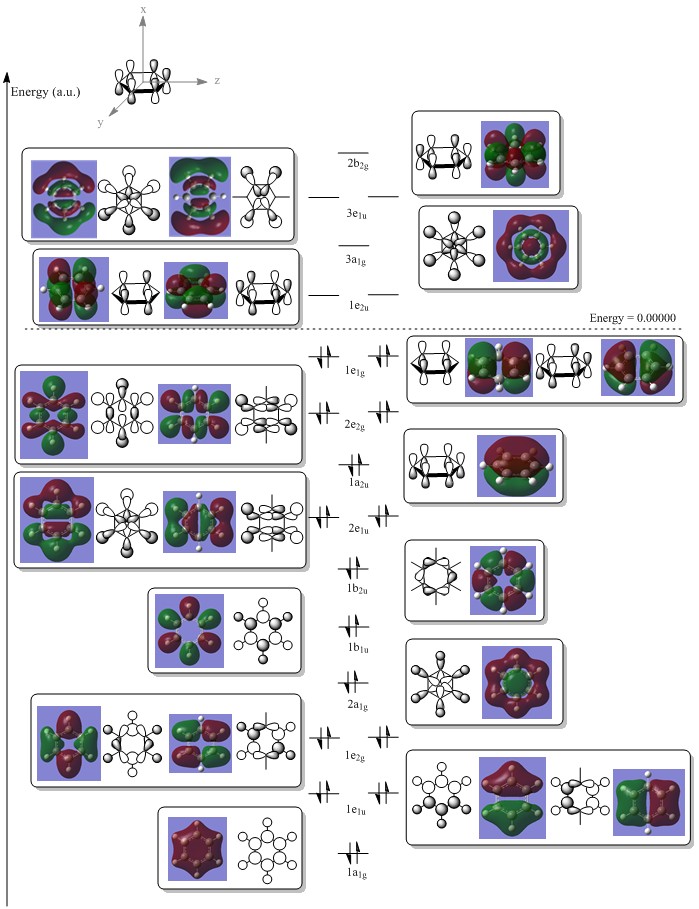
Benzene MO Diagram
The computed MOs in GaussView were used to construct the LCAO that come together to mix and as is seen in the MO diagram, the computed MOs resemble those predicted by LCAO very closely. It can be noted that there are some faults with the computed MOs, such as the orbitals can be very diffuse and thus overlap onto atoms which are not contributing to the molecular orbital, however in these cases the general nature of the orbital still remains correct and is consistent with LCAO theory.
Aromaticity can be derived and explained in several ways. The most widely used definition of aromaticity is a species which obeys Hückel’s Rule 5. In order for a molecule to be defined as aromatic there must be 4n+2 number of π electrons6 (where n is equal to 0 or any positive integer) found in a planar, cyclic, fully conjugated system. All of the compounds under investigation in this project have 6π electrons present in their structure, yielding n=1.
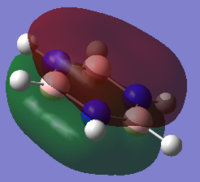
The MO that supports benzene’s aromaticity is the a2u MO which contains three fully bonding π MOs above and below the ring, which have overlapped sideways to form one large delocalised π system above and below the plane of the ring. This overlap can occur due to the cyclic nature of the sp2 hybridised molecule but also due to the close proximity and effective overlap of the p-orbitals on the ring. This delocalised π system also leads to a carbon-carbon bond length (140 pm)3 found between that of a typical carbon-carbon single bond (154 pm)3 and a carbon-carbon double bond (134 pm)3, further showing the effect the delocalised π system has on the molecule.
Comparison of MO's
| Benzene | Boratabenzene | Pyridinium | Borazine | |
|---|---|---|---|---|
| MO | 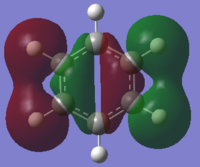
|

|
|
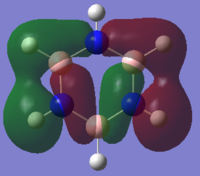
|
| LCAO | 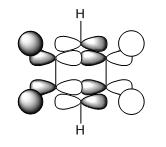
|
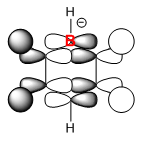
|
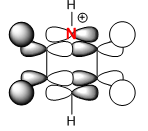
|
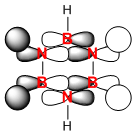
|
| Energy (a.u.) | -0.41653 | -0.20372 | -0.65086 | -0.43403 |
| Order | 15 | 14 | 16 | 14 |
| Degenerate (Yes/No)? | Yes | No | No | Yes |
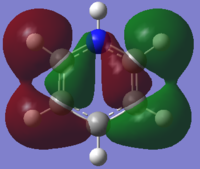
From the table above it can be seen that there is a strong in-phase through space bonding interaction between the s orbitals on the ortho and meta positioned hydrogen atoms and the p orbitals on the ortho and meta carbon atoms as well as the strong through space antibonding between the p orbitals on the two sets of ortho and meta ring atoms (either side of the molecule). The computed MO shows that benzene has a uniform distribution of the orbital which is expected due to its atomic and geomteric symmetry, however boratabenzene and pyridinium show some asymmetry in their computed MO's. In boratabenzene and pyridinium there is greater electron density found closer the most electronegative position of the molecule which in boratabenzene's case is the carbon situated furthest away from the boron atom in the para position whilst in pyridinium it is the nitrogen atom. The electronativity of the atoms in the molecule result in a tapering effect of the orbital towards the electronegative species due to inductive effects. This concept also applies to borazine as there is a greater difference in electronegativity between nitrogen and boron than of between boron or nitrogen and carbon, producing an MO with even more electron density found proximal to the nitrogen atoms.
The energies of the orbitals have been affected by substituting other heteroatoms into the benzene backbone to produce isoelectronic compounds. There is a clear stabilisation from the nitrogen atom in pyridinium and destabilisation from the boron atom in boratabenzene. However, the energy of borazine is expected to lie between this range, but lower than benzene as the opposing effects of the boron and nitrogen atoms cancel each other out, with nitrogen's stabilising effect being greater than boron's destabilising. Substitutions of heteroatoms results in the loss of degeneracy between particular MO's, which as a result causes the re-positioning/ordering of the energy levels and MOs. These orbitals under consideration are in the middle of the MO diagram and their positions are therefore affected by the loss of degeneracy of low-lying and higher lying orbitals, resulting in either a stabilisation or destabilisation in energy. In pyridinium and borazine it can be seen in the computed MOs that there may be some contribution by the p orbital on the nitrogen atom which is not predicted by LCAO. This prediction is also evident in the remaining orbitals making LCAO theory a a brief qualitative description of the MO's without having considered the point group of the molecule, its bond lengths and angles and many more good. Looking across all four calculated MOs there is a slight contribution on p orbitals suggesting LCAO theory should be used as a guide to help understand the more accurate calculated MOs.
| Benzene | Boratabenzene | Pyridinium | Borazine | |
|---|---|---|---|---|
| MO | 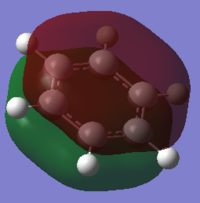
|
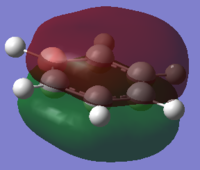
|
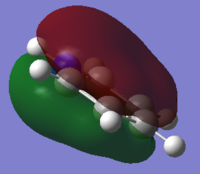
|
|
| LCAO | 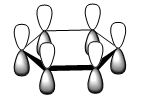
|
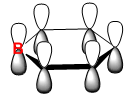
|
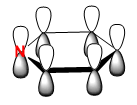
|
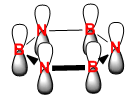
|
| Energy (a.u.) | -0.35994 | -0.13210 | -0.64062 | -0.36134 |
| Order | 17 | 17 | 17 | 17 |
| Degenerate (Yes/No)? | No | No | No | No |
The orbitals depicted in the table above show the delocalised π system above and below the plane of the ring. This sandwhich-like array only consists of px orbitals found above and below the plane of the ring which results in a strong through space bonding via a sideways overlap of the p-orbitals, with no antibonding component present. The series of MOs for this orbital across the series is very similar. However, there is a greater electron density (a more diffuse orbital) shown through the tendency of the orbital to swell in a particular region that is electronegative in nature. This effect can be seen clearly in borazine and pyridinium where the orbital has a sense of direction and tends to gather (be most dense) at electronegative regions.
The relative energies of the orbitals can be explained in a similar manner to those discussed above whereby the boron and nitrogen destabilise and stabilise respectively, due to electropositive species being higher in energy than species which are electronegative, making them good donors and acceptors respectively. Borazine is a highly symmetric molecule as mentioned in prior discussions, with a D3h point group, which as a result causes the electropositive and electronegative effects produced by boron and nitrogen to cancel one another out producing an energy of -0.36134 a.u.). The order of these orbitals remain the same as this is one of the many orbitals that contribute to the aromaticity of a molecule, suggesting that there are no degenerate orbitals present in each of the molecules in this region. In this particular case the LCAO theory doesn't allow one to predict/evaluate the contribution of each of the px orbitals to the molecular orbital.
| Benzene | Boratabenzene | Pyridinium | Borazine | |
|---|---|---|---|---|
| MO | 
|

|
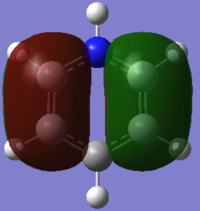
|

|
| LCAO | 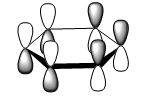
|
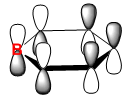
|
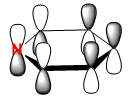
|
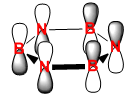
|
| Energy (a.u.) | -0.24690 | 0.01094 | -0.47886 | -0.27593 |
| Order | 21 | 21 | 21 | 21 |
| Degenerate (Yes/No)? | Yes | No | No | Yes |
The HOMO of benzene can be depicted with a LCAO of perpendicular p orbitals on each carbon atom of the ring, three being in phase and three being out of phase. This gives rise to the nodal plane perpendicular to the sigma framework. The energy of this orbital is computed to be -0.24690. It can be seen that the hydrogen 1s orbitals are not involved in these orbitals because they have the wrong symmetry. The energy of the boratabenzene HOMO is 0.01094, which is higher than the HOMO of benzene making this molecule more susceptible to electrophilic attack and it seems from the computed MO that the ordering of MO’s has perhaps changed. The same nodal planes however remain. The HOMO in pyridinium is at an energy of -0.47886 making this more stable than benzene - the electronegative nitrogen lowers the energy of the e1g orbital in which it is in. The lower energy of this homo makes it very unreactive towards electrophiles, as expected for a positively charged species. For the same reasons mentioned previously there is a loss of degeneracy of the HOMO in boratabenzene and pyridinium due to the lower symmetry state of these molecules (C2v).
There seems to be a trend in the relative energy of the MOs of benzene and its isoelectronic analogues in which a given orbital has the lowest energy in pyridinium followed by borazine, benzene and then boratabenzene. In all the molecules, the MO has one nodal plane which cuts the molecule in approximately two halves.
Effect of Substitution
Each of the different isoelectronic aromatic compounds under investigation differ in their symmetry point group depending on what atoms have been replaced on the carbon backbone of benzene. As molecular orbitals rely heavily on the symmetry of the molecule under investigation they will differ quite significantly between the structures and will have different symmetry labels.
| Compound | Point group |
|---|---|
| Benzene | D6h |
| Boratabenzene | C2v |
| Pyridinium | C2v |
| Borazine | D3h |
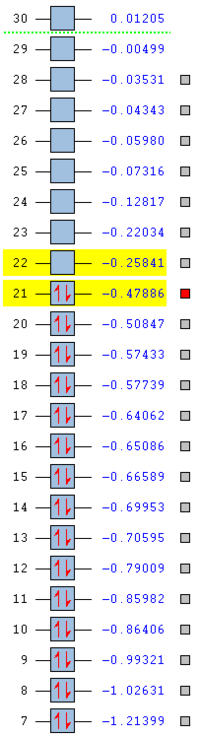
As the aromatic rings can be thought of a linear chain joined together at its terminal ends, benzene can be viewed as a repeat unit of 6 carbon atoms. Upon substitution of one of the carbon atoms by a boron atom or a nitrogen atom in the case of boratabenzene and pyridinium respectively the structure can no longer be viewed as a repeat unit due to the repositioning of the eigenvalued and thus the relative energies to produce a system that has no degeneracy. Borazine on the other hand can be seen to be made up of a repeat unit, allowing the presence of degeneracy in its MO energies. These properties are depicted in the table below.
| Benzene | Boratabenzene | Pyridinium | Borazine |
|---|---|---|---|
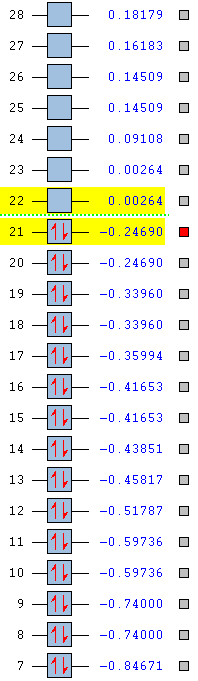
|
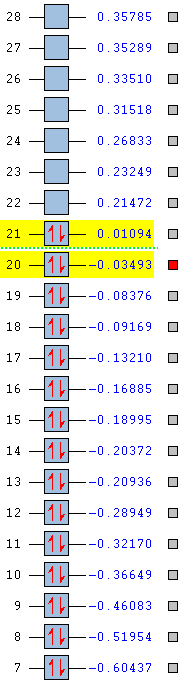
|
|

|
From the table above it can be concluded that the stability of the compound also depends on the type of atoms present in it, where the addition of a nitrogen atom increases the stability of the compound by lowering the HOMO and the LUMO, whereas the addition of an electropositive substituent makes the compound relatively unstable by raising the energy of the HOMO and the LUMO. From the table, one can say that stability increases from boratabenzene to pyridinium in the order of: pyridinium > borazine > benzene > boratabenzene. The two highly symmetric species, benzene and borazine are fairly similar in terms of stability due to the similarity in their HOMO energy levels and therefore MO shapes. Their stability can be explained as mentioned before in terms of repeat units, as the two structures contain repeat units the electron density in the molecule is more evenly spread, thus obeying the concept of aromaticity (uniform delocalisation). The reason why borazine is lower in energy than benzene is due to the fact the the presence of electronegative atoms outweighs the presence of electropositive atoms and overall lowers the energy of the MOs. Boratabenzene and pyrdinium however have a single boron atom or nitrogen atom in there structure respectively. The boron atom is electropositive, whilst the nitrogen atom is electronegative which results in a skewed distribution of electron density across the ring
Pyridinium is the most stable compound out of the four isoelectronic compounds with a HOMO at -0.47886 as a result of nitrogen's strong electronegativity and tendency to pull electron density towards itself. Not only is the HOMO lower in energy than benzene's HOMO (-0.24690), the LUMO of pyridinium is also lower in energy than benzene's HOMO, which as a result explains the reactivity of pyridinium whereby it is more resistant to attack by electrophiles. Finally, boratabenzene is the least stable structure under consideration with a HOMO at -0.03493 and a LUMO at 0.01094. The relative positions of these orbitals allows for effective electrophilic attack on the molecule, because the boron atom is very electropositive such that it raises the energy of the molecular orbitals.
References
1. P. Atkins, J. De Paula, Atkins' Physical Chemistry, Oxford University Press, 9th edn., 2010
2. S. Blanksby, G. Ellison, "Bond Dissociation Energies of Organic Molecules", Acc. Chem. Res., 2003,36, 255-263
3. Shriver & Atkins', Inorganic Chemistry, Oxford University Press, 5th edn., 2010,ch. 2, pp. 603
4. The Hunt Research Group website, http://www.huntresearchgroup.org.uk/teaching/year3_lab_start.html (accessed March 2015)
5. P. Rague von Schleyer, H. Jiao, Pure Appl. Chem., 1996, Vol. 68, No. 2, pp. 209-218, DOI: http://dx.doi.org/10.1351/pac199668020209
6. J. A. Berson, Chem. Int. Ed. Engl., 1996, Vol. 35, 23-34, pp. 2750-2764