Rep:Mod:AM01389684
BH3
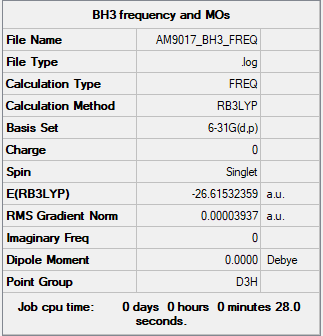
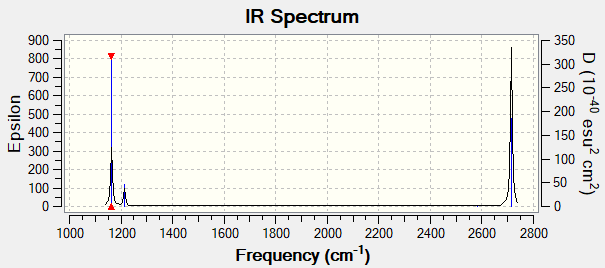
Item Value Threshold Converged? Maximum Force 0.000079 0.000450 YES RMS Force 0.000039 0.000300 YES Maximum Displacement 0.000310 0.001800 YES RMS Displacement 0.000155 0.001200 YES Low frequencies --- -0.3314 -0.1556 -0.0055 31.8788 33.4729 33.4746 Low frequencies --- 1163.3106 1213.3942 1213.3969
BH3 Molecule |
| Wavenumbers(cm-1) | Intensity(arbitrary units) | Symmetry | IR Active? | Type |
|---|---|---|---|---|
| 1163 | 93 | A2 | Yes | Out of plane bend |
| 1213 | 14 | E' | Yes | In plane bend |
| 1213 | 14 | E' | Yes | In plane bend |
| 2581 | 0 | A'1 | No | Symmetric stretch |
| 2714 | 126 | E' | Yes | Asymmetric stretch |
| 2714 | 126 | E' | Yes | Asymmetric stretch |
The table shows 6 vibrations however the IR spectrum only shows 3 peaks. This is because the vibrations at 1213 and 2714 cm-1 have degenerate vibrations (same energy) and therefore will still only produce one peak per set of degenerate vibrations. The vibration at 2581-1 shows no peak as it has 0 intensity due to the fact that it is a symmetric stretch and therefore has no change in dipole moment and so will not be IR active.
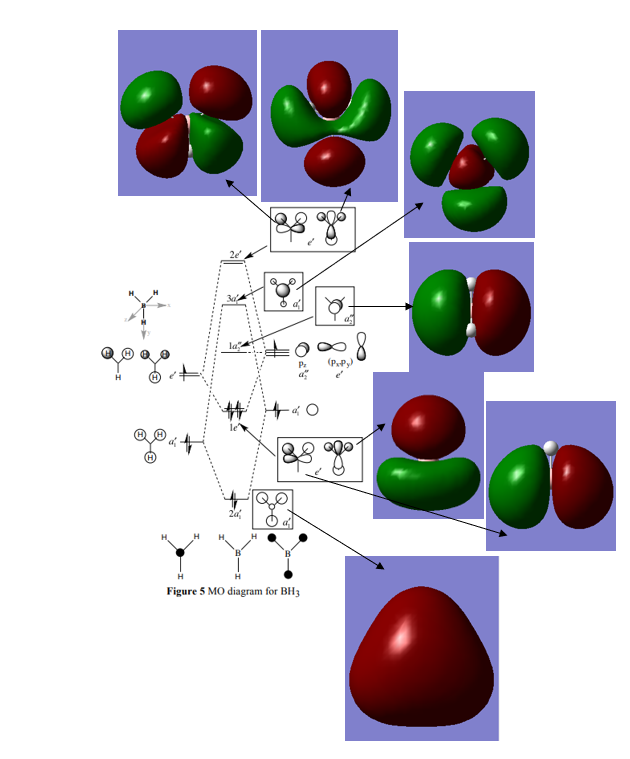
MO Diagram of BH3 with real orbitals
The picture shows the real orbitals to be more diffuse than the LCAOs therefore the electron density will cover more space than the LCAOs. This can be seen well in the 2e' orbitals for example.[1]
Ng611 (talk) 20:36, 29 May 2019 (BST) This is not really a significant difference. There are other more significant ones. Can you identify them?
NH3
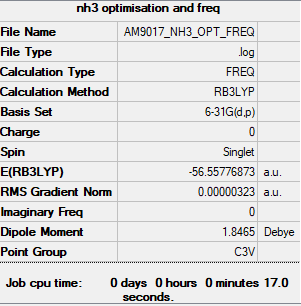
Item Value Threshold Converged?
Maximum Force 0.000006 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000014 0.001800 YES
RMS Displacement 0.000009 0.001200 YES
Low frequencies --- -0.0128 -0.0019 0.0007 7.1034 8.1048 8.1051
Low frequencies --- 1089.3834 1693.9368 1693.9368
NH3 Molecule |
NH3BH3
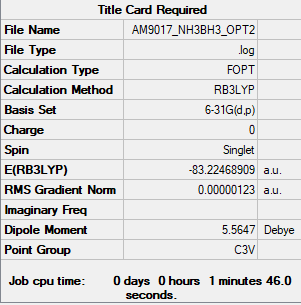
Item Value Threshold Converged? Maximum Force 0.000031 0.000450 YES RMS Force 0.000008 0.000300 YES Maximum Displacement 0.000165 0.001800 YES RMS Displacement 0.000086 0.001200 YES Low frequencies --- -0.1323 -0.0803 -0.0074 13.3999 13.4163 15.3144 Low frequencies --- 263.5208 633.1197 639.2499
NH3BH3 Molecule |
E(NH3) = -56.55777au
E(BH3) = -26.61532au
E(NH3BH3) = -83.22469au
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]
ΔE= -83.22469 - (-56.55777 - 26.61532)
ΔE= -0.05160au = -135KJ/mol. This value represents the formation of a B-N dative bond. This a relatively weak bond as when compared to a B-N covalent bond (389 KJ/mol) the value is much lower.[2]
Ng611 (talk) 20:36, 29 May 2019 (BST) Good!
NI3
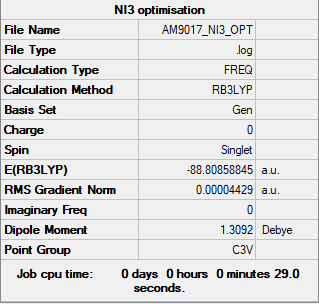
Item Value Threshold Converged?
Maximum Force 0.000102 0.000450 YES
RMS Force 0.000075 0.000300 YES
Maximum Displacement 0.000858 0.001800 YES
RMS Displacement 0.000629 0.001200 YES
Low frequencies --- -12.3847 -12.3783 -5.6131 -0.0040 0.0194 0.0711
Low frequencies --- 100.9307 100.9314 147.2333
Frequency file: Media:AM9017 NI3 OPT.LOG
NI3 Molecule |
The optimised N-I bond length was found to be 2.184Å.
[N(CH3)4)]+
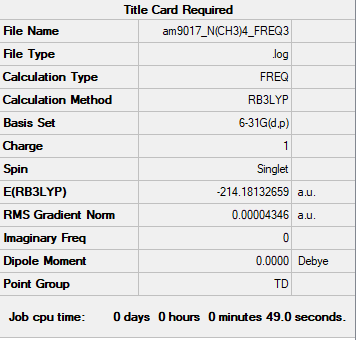
Item Value Threshold Converged? Maximum Force 0.000084 0.000450 YES RMS Force 0.000043 0.000300 YES Maximum Displacement 0.000835 0.001800 YES RMS Displacement 0.000477 0.001200 YES Low frequencies --- -0.0012 -0.0011 -0.0007 34.5465 34.5465 34.5465 Low frequencies --- 216.8630 316.1656 316.1656
Media:AM9017 N(CH3)4 FREQ3.LOG
[N(CH3)4]+ Molecule |
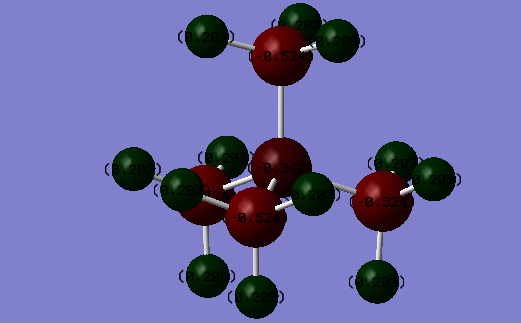
Charge Distributions
| Atom | Charge Distribution(Coulombs) |
|---|---|
| Hydrogen | 0.203 |
| Carbon | -0.524 |
| Nitrogen | -0.342 |
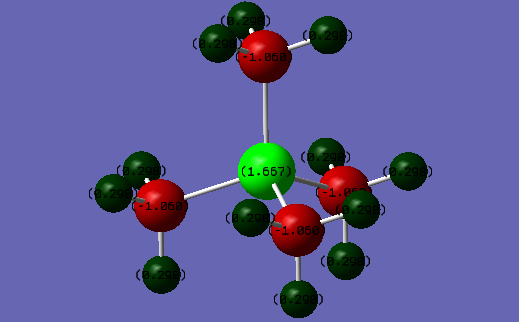
| Atom | Charge Distribution(Coulombs) |
|---|---|
| Hydrogen | 0.298 |
| Carbon | -1.060 |
| Phosphorous | 1.667 |
These two pictures show the charge distributions of [N(CH3)4)]+ and [P(CH3)4)]+ respectively. Carbon has an electronegativity of 2.55[3], which is higher than the phosphorous electronegativity of 2.19. The phosphorous atom is also larger and so the charge density will decrease which can stabilise a positive charge better than carbon. Therefore in the C-P bond, the carbon atom will have a more negative charge. However, nitrogen has an electronegativity of 3.04 which is higher than the carbon electronegativity but carbon has a more negative charge than nitrogen. This is because nitrogen forms 3 covalent bonds but since it only has 5 valence electrons the fourth bond will be a dative bond and so nitrogen will have a formal positive charge. Therefore the nitrogen will have a more positive charge than expected, if we were to just look at the electronegativities. The carbon atom in [N(CH3)4)]+ has a more positive charge than in [P(CH3)4)]+. This is because the formal positive charge is spread over the nitrogen and carbon atoms(instead of being located just on the nitrogen atom, as depicted in a traditional picture), especially since carbon has a lower electronegativity than nitrogen and so can stabilise a positive charge better. There is not much difference in the charge distributions of the hydrogen atoms of each molecule due to the fact that as the distance from the central atom increases, the effects of electronegativity will decrease.
Ng611 (talk) 20:38, 29 May 2019 (BST) Consider also the effect of molecular symmetry. Otherwise, good analysis.
A flaw of these pictures is that it shows the charges to be localised on each atom however in reality the charges are spread over the molecule. This can be seen by MO diagrams.
MO diagrams
| LCAO Diagram | MO | Description |
|---|---|---|
MO8 |
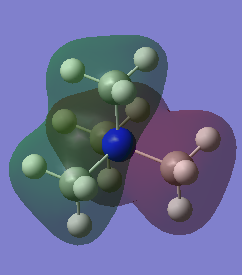 |
This ligand FO is comprised of in phase Hydrogen and Carbon s orbitals overlapping. The MO is then comprised of two in phase and two out of phase ligands FO which interact with the same phase lobes of the Nitrogen atom respectively. The only node is on the p orbital of the nitrogen atom and so is over a bonding orbital.
Ng611 (talk) 20:40, 29 May 2019 (BST) Your s-type FOs/p-orbital interaction is mislabelled here. |
MO10 |
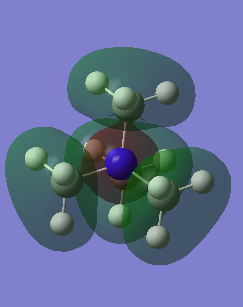 |
This is a bonding orbital. The only nodes are on the p orbitals on the carbon atoms. The p orbitals are orientated to be in phase with the nitrogen s orbital and so there are no nodes along the bonds.
Ng611 (talk) 20:41, 29 May 2019 (BST) This is actually antibonding, although the small 'nub' of electron density seems to suggest otherwise. You FOs are actually s-type. |
MO18 |
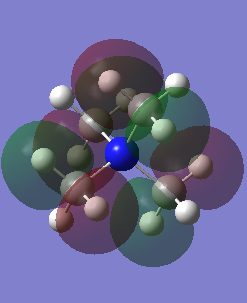 |
This is a non-bonding orbital. The nodes are on the p orbitals on the carbon atoms. The ligand orbitals here do not interact with the nitrogen atom. |
[P(CH3)4)]+
Item Value Threshold Converged? Maximum Force 0.000177 0.000450 YES RMS Force 0.000105 0.000300 YES Maximum Displacement 0.001509 0.001800 YES RMS Displacement 0.000874 0.001200 YES Low frequencies --- -0.0024 -0.0023 -0.0009 50.5667 50.5667 50.5667 Low frequencies --- 185.8154 210.8026 210.8026
Media:AM9017 -P(CH3)4- OPT FREQ.LOG
[P(CH3)4]+ Molecule |
Bibliography
- ↑ Prof. Hunt, P (2018), Lecture 4 Tutorial Problem Model Answers, Imperial College London.
- ↑ 1.Lange, N. & Dean, J. Lange's handbook of chemistry. (McGraw-Hill, 1979).
- ↑ Little, E. J., & Jones, M. M. (1960). A complete table of electronegativities. Journal of Chemical Education, 37(5), 231. https://doi.org/10.1021/ed037p231