Rep:Mod:AHall Comp2
Bonding and Molecular Orbitals in Main Group Compounds
Amh110 15:12, 18 February 2013 (UTC)
Optimisation of BH3
Using 3-21G basis set
BH3 was optimised using the B3LYP method with a 3-21G basis set. The optimised .log file can be found here.
- B-H bond length = 1.19349Å (lit.[1] 1.1900Å)
- H-B-H bond angle = 120.000°
- Total energy = -26.46226338 au (-69476.7 kJ/mol)

| File Name | BH3_opt |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 3-21G |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -26.46226338 a.u. |
| RMS Gradient Norm | 0.00020672 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Job cpu time: | 0 days 0 hours 0 minutes 20.0 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000413 0.000450 YES
RMS Force 0.000271 0.000300 YES
Maximum Displacement 0.001610 0.001800 YES
RMS Displacement 0.001054 0.001200 YES
Predicted change in Energy=-1.071764D-06
Optimization completed.
-- Stationary point found.
Using 6-31G(d,p) basis set
Using the output from the previous optimisation a further optimisation was performed using the B3LYP method with a 6-31G(d,p) basis set. The output .log file can be found here.
- B-H bond length = 1.19349Å
- H-B-H bond angle = 120.000°
- Total energy = -26.61722301 au (-69883.5 kJ/mol)
| File Name | BH3_OPT_631g_dp |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31+G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -26.61722301 a.u. |
| RMS Gradient Norm | 0.00014139 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Job cpu time: | 0 days 0 hours 0 minutes 10.0 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000283 0.000450 YES
RMS Force 0.000185 0.000300 YES
Maximum Displacement 0.001125 0.001800 YES
RMS Displacement 0.000736 0.001200 YES
Predicted change in Energy=-4.770182D-07
Optimization completed.
-- Stationary point found.
Pseudo-Potentials
Optimisation of TlBr3
The geometry of TlBr3 was optimised using a B3LYP method with a medium level basis set; LanL2DZ. The geometry of the TlBr3 was constrained so that only solutions with a D3h point group were calculated. A link to the optimised .log file can be found at DOI:10042/23345 .
- Tl-Br bond length = 2.65095 Å (lit.[1] 2.6182Å)
- Br-B-Br bond angle = 120.000°
- Total energy = -91.21812851 au
| File Name | TlBr3_opt_LanL2DZ |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | LANL2DZ |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -91.21812851 a.u. |
| RMS Gradient Norm | 0.00000090 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Job cpu time: | 0 days 0 hours 0 minutes 29.3 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000002 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000022 0.001800 YES
RMS Displacement 0.000014 0.001200 YES
Predicted change in Energy=-6.084070D-11
Optimization completed.
-- Stationary point found.
Optimisation of BBr3
Using the output from the 6-31G(d,p) optimised BH3 molecule, the hydrogen atoms were changed for bromine atoms. The molecule was then optimised using a 6-31G(d,p) basis set for the boron atom and a LanL2DZ basis set for the bromines. A link to the optimised .log file can be found at DOI:10042/23346 .
- B-Br bond length = 1.93396 Å (lit.[1] 1.893Å)
- Br-B-Br bond angle = 120.000°
- Total energy = -64.43645296 au
| File Name | BBr3_OPT_GEN |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | GEN |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -64.43645296 a.u. |
| RMS Gradient Norm | 0.00000382 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Job cpu time: | 0 days 0 hours 0 minutes 23.9 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000036 0.001800 YES
RMS Displacement 0.000023 0.001200 YES
Predicted change in Energy=-4.027020D-10
Optimization completed.
-- Stationary point found.
Comparison of Bond lengths
| Molecule | Bond Length/Å |
|---|---|
| BH3 | 1.19349 |
| BBr3 | 1.93396 |
| TlBr3 | 2.65095 |
NB. When BH3 is optimised with starting bond lengths 1.5Å the optimised bond length is 1.19349Å. If the structure is optimised starting from default bond length (1Å) then the optimised length is 1.19231Å.
The bond length for BH3 is approximately 0.74Å shorter than the bond length for BBr3. This can be explained by considering the differences in size of the ligands, as bromine is a much larger atom than hydrogen and so the distance between the boron and bromine nuclei will be much greater than the distance between the boron and hydrogen nuclei. As the bromine has very diffuse orbitals when compared to the hydrogen, interaction of the bromine and boron atoms will be weaker, also leading to a longer bond length. Both bromine and hydrogen atoms are one electron short of a full valence shell and so form a single covalent bond to boron, however the atoms are of very different sizes and so the bonds have different properties.
Changing the central atom to thallium also causes a large increase in bond length. Thallium is a much larger atom than boron and so again will cause a lengthening of the bond, as the valence electrons are much further from the nucleus and so the nuclei must be further apart to bond. There is a large size difference between thallium and bromine atoms, so the orbitals are much more diffuse for thallium than bromine, causing poorer interaction.
Gaussian shows bonds whenever two atoms are within a set distance which is predetermined from known bond lengths. In some cases Gaussian will not show a bond where one is expected to exist, as the atoms forming the bond are too far apart. This does not necessarily mean that there is no bonding interaction between the atoms as the visual representation supplied by the computer is only a model to illustrate the relative positions of the atoms, and does not show how the electrons are interacting in the molecule.
The concept of a physical bond is an inaccurate way of describing the interactions between atoms, which are better represented by showing the electronic interactions with molecular orbitals, however the concept of a bond is a useful approximation to simplify interactions within molecules. A covalent bond is where two atoms are held together by the presence of electron density between the atoms.
Frequency Calculations
BH3
Optimisation
A molecule of BH3 was optimised using the B3LYP method and the basis set 3-21G followed by a further optimisation using the basis set 6-31G(d,p). The optimised .log file can be found here.
- B-H bond length = 1.19231 Å (lit.[1] 1.1900Å)
- H-B-H bond angle = 120°
- Energy = -26.61532363 au.
| File Name | BH3_opt_6-31Gdp_2 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -26.61532363 a.u. |
| RMS Gradient Norm | 0.00000475 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Job cpu time: | 0 days 0 hours 0 minutes 11.0 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000009 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000038 0.001800 YES
RMS Displacement 0.000025 0.001200 YES
Predicted change in Energy=-5.342736D-10
Optimization completed.
-- Stationary point found.
Frequency Analysis
Frequency analysis was performed using the B3LYP method and the basis set 6-31G(b,p). The energy after frequency analysis was the same as for the geometry optimisation. The .log file can be found here.
| File Name | AHall_bh3_freq_2 |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -26.61532363 a.u. |
| RMS Gradient Norm | 0.00000477 a.u. |
| Imaginary Freq | 0 |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Job cpu time: | 0 days 0 hours 0 minutes 14.0 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000010 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000038 0.001800 YES
RMS Displacement 0.000019 0.001200 YES
Predicted change in Energy=-5.368808D-10
Optimization completed.
-- Stationary point found.
Low frequencies --- -3.5991 -1.1355 -0.0054 1.3745 9.7046 9.7707
Low frequencies --- 1162.9825 1213.1733 1213.1760
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A2" E' E'
Frequencies -- 1162.9825 1213.1733 1213.1760
Red. masses -- 1.2531 1.1072 1.1072
Frc consts -- 0.9986 0.9601 0.9601
IR Inten -- 92.5497 14.0545 14.0581
Atom AN X Y Z X Y Z X Y Z
1 5 0.00 0.00 0.16 0.00 0.10 0.00 -0.10 0.00 0.00
2 1 0.00 0.00 -0.57 0.00 0.08 0.00 0.81 0.00 0.00
3 1 0.00 0.00 -0.57 0.39 -0.59 0.00 0.14 -0.39 0.00
4 1 0.00 0.00 -0.57 -0.39 -0.59 0.00 0.14 0.39 0.00
4 5 6
A1' E' E'
Frequencies -- 2582.3247 2715.4994 2715.5006
Red. masses -- 1.0078 1.1273 1.1273
Frc consts -- 3.9597 4.8979 4.8979
IR Inten -- 0.0000 126.3285 126.3189
Atom AN X Y Z X Y Z X Y Z
1 5 0.00 0.00 0.00 0.11 0.00 0.00 0.00 0.11 0.00
2 1 0.00 -0.58 0.00 0.02 0.00 0.00 0.00 -0.81 0.00
3 1 0.50 0.29 0.00 -0.60 -0.36 0.00 -0.36 -0.19 0.00
4 1 -0.50 0.29 0.00 -0.60 0.36 0.00 0.36 -0.19 0.00
The second and third and the fifth and sixth vibrations are very close in frequency, and so are overlapped on the spectrum leading to only three peaks being visible on the spectrum.
TlBr3
Frequency analysis was carried out on TlBr3 using the previously optimised .log file as the input. The output .log file can be found at: DOI:10042/23395 .
| File Name | AHall_TlBr3_freq |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | LANL2DZ |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -91.21812851 a.u. |
| RMS Gradient Norm | 0.00000088 a.u. |
| Imaginary Freq | 0 |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Job cpu time: | 0 days 0 hours 0 minutes 22.2 seconds. |
Item Value Threshold Converged? Maximum Force 0.000002 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000022 0.001800 YES RMS Displacement 0.000011 0.001200 YES
Low frequencies --- -3.4213 -0.0026 -0.0004 0.0015 3.9367 3.9367
Low frequencies --- 46.4289 46.4292 52.1449
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
E' E' A2"
Frequencies -- 46.4289 46.4292 52.1449
Red. masses -- 88.4613 88.4613 117.7209
Frc consts -- 0.1124 0.1124 0.1886
IR Inten -- 3.6867 3.6867 5.8466
Atom AN X Y Z X Y Z X Y Z
1 81 0.00 0.28 0.00 -0.28 0.00 0.00 0.00 0.00 0.55
2 35 0.00 0.26 0.00 0.74 0.00 0.00 0.00 0.00 -0.48
3 35 0.43 -0.49 0.00 -0.01 -0.43 0.00 0.00 0.00 -0.48
4 35 -0.43 -0.49 0.00 -0.01 0.43 0.00 0.00 0.00 -0.48
4 5 6
A1' E' E'
Frequencies -- 165.2685 210.6948 210.6948
Red. masses -- 78.9183 101.4032 101.4032
Frc consts -- 1.2700 2.6522 2.6522
IR Inten -- 0.0000 25.4830 25.4797
Atom AN X Y Z X Y Z X Y Z
1 81 0.00 0.00 0.00 0.42 0.00 0.00 0.00 0.42 0.00
2 35 0.00 -0.58 0.00 0.01 0.00 0.00 0.00 -0.74 0.00
3 35 0.50 0.29 0.00 -0.55 -0.32 0.00 -0.32 -0.18 0.00
4 35 -0.50 0.29 0.00 -0.55 0.32 0.00 0.32 -0.18 0.00
The lowest 'real' mode is 46.4289cm-1, approximately 10 times greater than the highest 'zero' frequency mode.
Comparison
| Number | BH3 | TlBr3 | ||
|---|---|---|---|---|
| Frequency /cm-1 | Symmetry | Frequency /cm-1 | Symmetry | |
| 1 | 1162.98 | A2" | 46.43 | E' |
| 2 | 1213.17 | E' | 46.43 | E' |
| 3 | 1213.18 | E' | 52.14 | A2" |
| 4 | 2582.32 | A1' | 165.27 | A1' |
| 5 | 2715.50 | E' | 210.69 | E' |
| 6 | 2715.50 | E' | 210.69 | E' |
There is a large difference in the stretching frequencies for BH3 and TlBr3, which reflects the difference in bond strength. As the BH3 has higher frequency vibrations the energy needed to cause the vibrations is higher, indicating a stronger bond. This increased bond strength results in the B-H bond being much shorter than the Tl-Br bond.
For TlBr3 the out-of-plane bending mode is the third vibration observed, whereas for BH3 this mode is the first observed. As the energies of the modes are fairly similar, the change required to cause this reordering does not need to be very large.
Both spectra show a single peak at higher frequencies and two peaks close together at lower frequencies, with the single peak being of much higher intensity than the two lower peaks. The separation between the peaks is much greater for the BH3 than for the TlBr3. The vibrations can be divided into two sets; vibrations 1-3 which have lower frequency, and vibrations 4-6 which have higher frequency. The first set of vibrations are due to bending of the bonds, whereas the second set are due to bond stretching. As it takes more energy to stretch a bond than to bend it, the second set of vibrations are at higher frequencies.
It is important to use the same method and basis set for optimisation and frequency analysis calculations as otherwise the results will be incorrect due to differences in the optimised structure using different basis sets.
Frequency analysis is carried out to check that the optimisation has reached a minimum, as if the optimisation is not complete then the low frequencies will be large compared to the 'real' frequencies, and there is a chance that some of the 'real' frequencies will be negative, which is impossible.
The low frequencies represent the motion of the centre of mass of the molecule, and are represented by the '-6' part of the 3N-6 vibrational modes for a molecule with 'N' atoms.
MOs
BH3
The checkpoint file (.chk) from the optimised BH3 molecule was submitted to Energy calculation to calculate the MOs. The output checkpoint file can be found at: DOI:10042/23403 .
| File Name | AHall_bh3_MOs |
| File Type | .fch |
| Calculation Type | SP |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(D,P) |
| Charge | 0 |
| Spin | Singlet |
| Total Energy | -26.61532363 a.u. |
| RMS Gradient Norm | 0.00000000 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group |
There is a close relationship between the LCAO predicted orbitals and the calculated MOs. The calculated MOs show what happens when the combined atomic orbitals mix producing orbitals which are delocalised over multiple atoms. Some of the anti-bonding MOs (particularly 2e'1) have lobes which are bent away from the shape predicted by LCAO due to repulsion with other lobes.
This implies that MO theory is a good representation of the position of the electrons in the molecules, as it matches well to the predicted orbitals from LCAO. The calculated MOs are better than the LCAO orbitals as they show how the orbitals mix to give bonding and anti-bonding orbitals and so can help with theory of how the bonding in the molecule works.
NBO Analysis
NH3
Optimisation
A molecule of NH3 was optimised using the B3LYP method with a 6-31G(d,p) basis set. The .log file can be found here.
| File Name | NH3_opt |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -56.55776856 a.u. |
| RMS Gradient Norm | 0.00000885 a.u. |
| Imaginary Freq | |
| Dipole Moment | 1.8464 Debye |
| Point Group | C1 |
| Job cpu time: | 0 days 0 hours 0 minutes 25.0 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000024 0.000450 YES
RMS Force 0.000012 0.000300 YES
Maximum Displacement 0.000079 0.001800 YES
RMS Displacement 0.000053 0.001200 YES
Predicted change in Energy=-1.629732D-09
Optimization completed.
-- Stationary point found.
Frequency Analysis
The optimised NH3 .log file was submitted to frequency analysis. The output .log file can be found here.

Low frequencies --- -30.7295 0.0008 0.0014 0.0016 20.1705 28.2664
Low frequencies --- 1089.5535 1694.1244 1694.1856
Harmonic frequencies (cm**-1), IR intensities (KM/Mole), Raman scattering
activities (A**4/AMU), depolarization ratios for plane and unpolarized
incident light, reduced masses (AMU), force constants (mDyne/A),
and normal coordinates:
1 2 3
A A A
Frequencies -- 1089.5535 1694.1244 1694.1856
Red. masses -- 1.1800 1.0644 1.0644
Frc consts -- 0.8253 1.8000 1.8001
IR Inten -- 145.4405 13.5558 13.5560
Atom AN X Y Z X Y Z X Y Z
1 7 0.12 0.00 0.00 0.00 -0.02 -0.06 0.00 0.06 -0.02
2 1 -0.53 -0.21 0.00 -0.07 -0.04 0.73 0.25 0.14 0.20
3 1 -0.53 0.11 0.18 0.25 -0.24 -0.03 -0.07 -0.62 0.40
4 1 -0.53 0.11 -0.18 -0.18 0.52 0.18 -0.18 -0.41 -0.36
4 5 6
A A A
Frequencies -- 3460.9818 3589.3998 3589.5230
Red. masses -- 1.0272 1.0883 1.0883
Frc consts -- 7.2496 8.2615 8.2621
IR Inten -- 1.0593 0.2700 0.2709
Atom AN X Y Z X Y Z X Y Z
1 7 -0.04 0.00 0.00 0.00 -0.04 0.07 0.00 0.07 0.04
2 1 0.18 -0.55 0.00 -0.15 0.35 0.02 0.27 -0.66 0.01
3 1 0.18 0.27 0.47 -0.16 -0.22 -0.34 -0.26 -0.31 -0.56
4 1 0.18 0.27 -0.48 0.31 0.38 -0.65 -0.01 0.01 0.03
Item Value Threshold Converged?
Maximum Force 0.000021 0.000450 YES
RMS Force 0.000009 0.000300 YES
Maximum Displacement 0.000078 0.001800 YES
RMS Displacement 0.000039 0.001200 YES
Predicted change in Energy=-1.611689D-09
Optimization completed.
-- Stationary point found.
As the low frequencies are significantly lower than the main frequencies the optimisation is complete.
Molecular Orbitals
A link to the .chk file for the energy calculation can be found at: DOI:10042/23452 .
 |
 |
 |
 |
 |
 |
 |

|
| MO 1 | MO 2 | MO 3 | MO 4 (HOMO) | MO 5 (LUMO) | MO 6 | MO 7 | MO 8 |
NBO analysis

The charge distribution of NH3 is indicated on the diagram to the left. The charge on nitrogen is -1.125 and the charge on hydrogen is 0.375
Summary of Natural Population Analysis:
Natural Population
Natural -----------------------------------------------
Atom No Charge Core Valence Rydberg Total
-----------------------------------------------------------------------
N 1 -1.12515 1.99982 6.11104 0.01429 8.12515
H 2 0.37505 0.00000 0.62250 0.00246 0.62495
H 3 0.37505 0.00000 0.62250 0.00246 0.62495
H 4 0.37505 0.00000 0.62249 0.00246 0.62495
=======================================================================
* Total * 0.00000 1.99982 7.97852 0.02166 10.00000
(Occupancy) Bond orbital/ Coefficients/ Hybrids
---------------------------------------------------------------------------------
1. (1.99909) BD ( 1) N 1 - H 2
( 68.83%) 0.8297* N 1 s( 24.87%)p 3.02( 75.05%)d 0.00( 0.09%)
-0.0001 -0.4986 -0.0059 0.0000 -0.2910
0.0052 0.8155 0.0277 0.0000 0.0000
0.0281 0.0000 0.0000 0.0032 0.0082
( 31.17%) 0.5583* H 2 s( 99.91%)p 0.00( 0.09%)
-0.9996 0.0000 0.0072 -0.0289 0.0000
2. (1.99909) BD ( 1) N 1 - H 3
( 68.83%) 0.8297* N 1 s( 24.86%)p 3.02( 75.05%)d 0.00( 0.09%)
0.0001 0.4986 0.0059 0.0000 0.2910
-0.0052 0.4077 0.0138 0.7062 0.0240
0.0140 0.0243 0.0076 0.0033 0.0031
( 31.17%) 0.5583* H 3 s( 99.91%)p 0.00( 0.09%)
0.9996 0.0000 -0.0072 -0.0145 -0.0250
3. (1.99909) BD ( 1) N 1 - H 4
( 68.83%) 0.8297* N 1 s( 24.87%)p 3.02( 75.05%)d 0.00( 0.09%)
0.0001 0.4986 0.0059 0.0000 0.2909
-0.0052 0.4077 0.0138 -0.7062 -0.0239
0.0140 -0.0243 -0.0076 0.0033 0.0031
( 31.17%) 0.5583* H 4 s( 99.91%)p 0.00( 0.09%)
0.9996 0.0000 -0.0072 -0.0145 0.0250
4. (1.99982) CR ( 1) N 1 s(100.00%)
1.0000 -0.0002 0.0000 0.0000 0.0000
0.0000 0.0000 0.0000 0.0000 0.0000
0.0000 0.0000 0.0000 0.0000 0.0000
5. (1.99721) LP ( 1) N 1 s( 25.38%)p 2.94( 74.52%)d 0.00( 0.10%)
0.0001 0.5036 -0.0120 0.0000 -0.8618
0.0505 0.0000 0.0000 0.0000 0.0000
0.0000 0.0000 0.0000 -0.0269 0.0155
6. (0.00000) RY*( 1) N 1 s( 99.98%)p 0.00( 0.02%)d 0.00( 0.00%)
7. (0.00000) RY*( 2) N 1 s(100.00%)
8. (0.00000) RY*( 3) N 1 s( 0.03%)p99.99( 99.97%)d 0.01( 0.00%)
9. (0.00000) RY*( 4) N 1 s( 0.00%)p 1.00(100.00%)d 0.00( 0.00%)
10. (0.00000) RY*( 5) N 1 s( 0.00%)p 1.00(100.00%)d 0.00( 0.00%)
11. (0.00000) RY*( 6) N 1 s( 0.00%)p 1.00( 0.12%)d99.99( 99.88%)
12. (0.00000) RY*( 7) N 1 s( 0.00%)p 1.00( 0.12%)d99.99( 99.88%)
13. (0.00000) RY*( 8) N 1 s( 0.00%)p 1.00( 0.01%)d99.99( 99.99%)
14. (0.00000) RY*( 9) N 1 s( 0.01%)p 4.42( 0.06%)d99.99( 99.92%)
15. (0.00000) RY*(10) N 1 s( 0.00%)p 1.00( 0.03%)d99.99( 99.97%)
16. (0.00112) RY*( 1) H 2 s( 72.77%)p 0.37( 27.23%)
0.0038 0.8531 0.5218 -0.0017 -0.0001
17. (0.00045) RY*( 2) H 2 s( 26.59%)p 2.76( 73.41%)
-0.0017 0.5157 -0.8435 -0.1501 -0.0007
18. (0.00034) RY*( 3) H 2 s( 0.00%)p 1.00(100.00%)
0.0000 0.0004 -0.0005 -0.0001 1.0000
19. (0.00000) RY*( 4) H 2 s( 0.72%)p99.99( 99.28%)
20. (0.00112) RY*( 1) H 3 s( 72.77%)p 0.37( 27.23%)
0.0038 0.8531 0.5218 0.0009 0.0014
21. (0.00045) RY*( 2) H 3 s( 26.59%)p 2.76( 73.41%)
-0.0017 0.5157 -0.8435 0.0756 0.1297
22. (0.00034) RY*( 3) H 3 s( 0.00%)p 1.00(100.00%)
0.0000 0.0004 -0.0005 -0.8660 0.5000
23. (0.00000) RY*( 4) H 3 s( 0.72%)p99.99( 99.28%)
24. (0.00112) RY*( 1) H 4 s( 72.76%)p 0.37( 27.24%)
0.0038 0.8530 0.5219 0.0009 -0.0015
25. (0.00045) RY*( 2) H 4 s( 26.61%)p 2.76( 73.39%)
-0.0017 0.5158 -0.8435 0.0750 -0.1300
26. (0.00034) RY*( 3) H 4 s( 0.00%)p 1.00(100.00%)
0.0000 0.0000 0.0000 0.8660 0.5000
27. (0.00000) RY*( 4) H 4 s( 0.72%)p99.99( 99.28%)
28. (0.00000) BD*( 1) N 1 - H 2
( 31.17%) 0.5583* N 1 s( 24.87%)p 3.02( 75.05%)d 0.00( 0.09%)
( 68.83%) -0.8297* H 2 s( 99.91%)p 0.00( 0.09%)
29. (0.00000) BD*( 1) N 1 - H 3
( 31.17%) 0.5583* N 1 s( 24.86%)p 3.02( 75.05%)d 0.00( 0.09%)
( 68.83%) -0.8297* H 3 s( 99.91%)p 0.00( 0.09%)
30. (0.00000) BD*( 1) N 1 - H 4
( 31.17%) 0.5583* N 1 s( 24.87%)p 3.02( 75.05%)d 0.00( 0.09%)
( 68.83%) -0.8297* H 4 s( 99.91%)p 0.00( 0.09%)
Natural Bond Orbitals (Summary):
Principal Delocalizations
NBO Occupancy Energy (geminal,vicinal,remote)
====================================================================================
Molecular unit 1 (H3N)
1. BD ( 1) N 1 - H 2 1.99909 -0.60417
2. BD ( 1) N 1 - H 3 1.99909 -0.60417
3. BD ( 1) N 1 - H 4 1.99909 -0.60416
4. CR ( 1) N 1 1.99982 -14.16768
5. LP ( 1) N 1 1.99721 -0.31756 24(v),16(v),20(v),17(v)
21(v),25(v)
Association Energies
Optimisation
A molecule of BH3NH3 was created and optimised using the B3LYP method with a 3-21G basis set: (.log file is at DOI:10042/23512 )
| File Name | AHall_NB_3_21 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 3-21G |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -82.76661837 a.u. |
| RMS Gradient Norm | 0.00003006 a.u. |
| Imaginary Freq | |
| Dipole Moment | 5.8431 Debye |
| Point Group | C1 |
| Job cpu time: | 0 days 0 hours 1 minutes 9.7 seconds. |
Item Value Threshold Converged? Maximum Force 0.000094 0.000450 YES RMS Force 0.000030 0.000300 YES Maximum Displacement 0.000419 0.001800 YES RMS Displacement 0.000178 0.001200 YES Predicted change in Energy=-5.742850D-08 Optimization completed.
The molecule was they further optimised using the 6-31G(d,p) basis set: (.log file is at DOI:10042/23514 )
| File Name | AHall_NB_6_31 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -82.22468922 a.u. |
| RMS Gradient Norm | 0.00005766 a.u. |
| Imaginary Freq | |
| Dipole Moment | 5.5623 Debye |
| Point Group | C1 |
| Job cpu time: | 0 days 0 hours 1 minutes 8.1 seconds. |
Item Value Threshold Converged? Maximum Force 0.000134 0.000450 YES RMS Force 0.000037 0.000300 YES Maximum Displacement 0.001246 0.001800 YES RMS Displacement 0.000556 0.001200 YES Predicted change in Energy=-1.230457D-07 Optimization completed.
Frequency Analysis
Frequency analysis was performed on the optimised NH3BH3 molecule. The output .log file can be found at DOI:10042/23520 .
| File Name | AHall_NB_freq |
| File Type | .log |
| Calculation Type | FREQ |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -82.22468920 a.u. |
| RMS Gradient Norm | 0.00005767 a.u. |
| Imaginary Freq | |
| Dipole Moment | 5.5623 Debye |
| Point Group | C1 |
| Job cpu time: | 0 days 0 hours 1 minutes 5.0 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000260 0.000450 YES
RMS Force 0.000058 0.000300 YES
Maximum Displacement 0.001437 0.001800 YES
RMS Displacement 0.000712 0.001200 YES
Predicted change in Energy=-2.209903D-07
Optimization completed.
-- Stationary point found.

| Frequency | Intensity | Frequency | Intensity | |
|---|---|---|---|---|
| 262.67 | 0.0000 | 1329.09 | 113.7119 | |
| 631.28 | 14.1300 | 1676.07 | 27.5527 | |
| 637.66 | 3.5747 | 1676.13 | 27.5350 | |
| 638.79 | 3.5764 | 2472.42 | 67.0654 | |
| 1068.50 | 40.4761 | 2532.75 | 231.1535 | |
| 1068.96 | 40.5518 | 2532.90 | 231.1579 | |
| 1196.15 | 109.0108 | 3464.04 | 2.5184 | |
| 1203.28 | 3.5024 | 3580.87 | 27.8921 | |
| 1203.57 | 3.4862 | 3580.97 | 27.9013 |
Low frequencies --- -14.4083 0.0011 0.0012 0.0014 12.1717 17.1399 Low frequencies --- 262.6686 631.2804 637.6627
Comparison
Energies:
- NH3 = -56.55776856 au.
- BH3 = -26.61532363 au.
- NH3BH3 = -83.22468920 au.
Energy difference between Products and Reagents = (-83.22468920) - (-56.5577685) - (-26.61532363) = -0.05159707 au. Convert to kJmol-1: -0.05159707 x 2625.50 = -135.455 kJmol-1 (3d.p.)
Dissociation energy = 135.455 kJmol-1 (lit.[2] 31.7 kcal/mol (=132.63 kJ/mol))
Aromaticity
Benzene
Optimisation
A molecule of benzene was optimised using the B3LYP basis set and the 3-21G basis set followed by the 6-31G(d,p) basis set. The output .log files can be found at DOI:10042/23541 and DOI:10042/23543
| File Name | AHall_ben_opt_6_31 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -232.25819628 a.u. |
| RMS Gradient Norm | 0.00004042 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group | C1 |
| Job cpu time: | 0 days 0 hours 2 minutes 9.2 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000090 0.000450 YES
RMS Force 0.000024 0.000300 YES
Maximum Displacement 0.000337 0.001800 YES
RMS Displacement 0.000103 0.001200 YES
Predicted change in Energy=-4.743663D-08
Optimization completed.
-- Stationary point found.
As the point group is incorrectly assigned to be C1, the final optimization was repeated with the point group constrained to D6h. The output .log files can be found at DOI:10042/23671 .
| File Name | AHall_ben_D6h |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -232.25821426 a.u. |
| RMS Gradient Norm | 0.00004040 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group | D6H |
| Job cpu time: | 0 days 0 hours 0 minutes 18.1 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000084 0.000450 YES
RMS Force 0.000029 0.000300 YES
Maximum Displacement 0.000182 0.001800 YES
RMS Displacement 0.000075 0.001200 YES
Predicted change in Energy=-4.892354D-08
Optimization completed.
-- Stationary point found.
Frequency Analysis

Frequency analysis was performed on the optimised benzene molecule. The .log file can be found at DOI:10042/23673 . The low frequencies are sufficiently low that the optimisation is complete:
Low frequencies --- -0.0088 -0.0041 -0.0041 11.3041 11.3041 15.5388 Low frequencies --- 414.2825 414.2825 621.2723
MOs
The energy of the benzene was optimised and MOs calculated. The formatted checkpoint file can be found at DOI:10042/23675 .
The calculated MOs were analysed and a MO diagram produced for the six MOs on either side of the HOMO/LUMO gap:

NBO analysis

NBO analysis was carried out as part of the energy calculations. The carbon atoms have a charge of -0.23859 and the hydrogen atoms +0.23859 and the molecule is completely symmetrical.
The analysis also shows that the C-C bonds are formed from equal contributions of the orbitals of each carbon. The C-C bond is formed of 35% 's' atomic orbitals and 65% 'p' atomic orbitals, implying that the bonding is sp2 as expected. There is also a very small contribution from 'd' orbitals (0.04%). The π bonding is shown to be formed almost entirely of 'p' orbitals, with a very small 'd' orbital contribution (0.04%). As expected the π bonding also has equal contributions from each carbon atom.
The C-H bonds contain unequal contributions from the carbon (62%) and the hydrogen (38%). The carbon contribution to the bonding is 30% 's' and 70% 'p' with a very small amount of 'd' contribution (0.04%), implying a hybridisation close to sp2. The hydrogen contribution to the bonding is almost entirely due to 's' orbitals, with a very small amount of 'p' contribution (0.05%).
The core orbitals on carbon are shown to be entirely 's' orbitals.
The Second Order Perturbation Theory Analysis of Fock Matrix in NBO Basis table shows that E(2) is 20.43 kcal/mol for the interactions between the 'p' based C-C bonds, implying that there is a large amount of mixing between these orbitals and so supporting the idea of a delocalised 'p' electron cloud in aromatic molecules.
The energy of the bonding orbitals is around -0.2 to -0.6 au. whereas the core orbitals have energies of around -10 au. and so will not affect the interactions with the valence electrons.
Boratabenzene
Optimisation
Optimisation was performed on a molecule of Boratabenzene (BC5H6) using the B3lYP method with a 3-21G basis set followed by a 6-31G(d,p) basis set. As the point group was incorrectly determined to be C1 the second optimisation was repeated with the point group constrained to C2v. The optimised .log file can be found at DOI:10042/23710 .
| File Name | AHall_bor_opt_c2v |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | -1 |
| Spin | Singlet |
| E(RB3LYP) | -219.02053052 a.u. |
| RMS Gradient Norm | 0.00003502 a.u. |
| Imaginary Freq | |
| Dipole Moment | 2.8454 Debye |
| Point Group | C2V |
| Job cpu time: | 0 days 0 hours 0 minutes 23.4 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000062 0.000450 YES
RMS Force 0.000018 0.000300 YES
Maximum Displacement 0.000218 0.001800 YES
RMS Displacement 0.000071 0.001200 YES
Predicted change in Energy=-3.182072D-08
Optimization completed.
-- Stationary point found.
Frequency Analysis

As the low frequencies are low compared to the 'real' frequencies, and none of the 'real' frequencies are negative the optimisation was successful. A link to the .log file can be found at DOI:10042/23724 .
Low frequencies --- -12.4155 -0.0008 -0.0003 0.0006 14.0145 18.0525 Low frequencies --- 371.3796 404.1483 565.1933
MOs
The MOs for boratabenzene are shown below. A link to the relevant files can be found at DOI:10042/23723 .
The presence of the boron atom strongly disrupts the shape of the MOs; for some MOs the boron atom contributes more to the MO than the carbon atoms (eg. MOs 16, 18, 19, 21), and for some MOs the boron is contributing less to the MO (eg. 17, 24, 28). This can be explained by considering the difference in electronegativity between carbon and boron. As the boron is more electropositive than carbon (and hydrogen) the atomic orbitals of boron will be higher in energy than those for carbon. This means that the boron contributes less to bonding interactions than to anti-bonding interactions; for example in MO 17, all of the interactions are bonding, so the boron contributes less, and for MO 23 the interactions are anti-bonding so the boron contributes more.

MO 16 |

MO 17 |
MO 18 |

MO 19 |
MO 20 |
MO 21 |

MO 22 |
MO 23 |

MO 24 |

MO 25 |
MO 26 |
MO 27 |

MO 28 |
NBO anaylsis

The analysis also shows that the C-C bonds are formed from nearly equal contributions of the orbitals of each carbon. The C-C bond is formed of 36% 's' atomic orbitals and 64% 'p' atomic orbitals, implying that the bonding is close to sp2. There is also a very small contribution from 'd' orbitals (0.04%). The π bonding is shown to be formed almost entirely of 'p' orbitals, with a very small 'd' orbital contribution (0.03%). As expected the π bonding also has equal contributions from each carbon atom.
The C-H bonds vary considerable around the ring (unlike in benzene where they are all equal) with some cases the carbon being close to sp2 (70% 'p', 30% 's') and in others (closer to the boron) the carbon is nearly sp3 (74% 'p', 25% 's'). The carbon generally contributes to about 60% with the remainder being made up by the hydrogen 's' orbital.
The C-B bond is formed from about 66% carbon (42% 's', 58% 'p') and 33% boron (33% 's', 67% 'p'). The B-H bond is 55% hydrogen and 45% boron, with the boron atom as sp2 (33% 's', 67% 'p') and the hydrogen entirely 's'.
The core orbitals on carbon and boron are shown to be entirely 's' orbitals.
Lone pairs have been assigned to the boron and the carbon directly opposite the boron, both with entirely 'p' character.
Significant mixing is observed for the π bonds on carbon, with a small amount of mixing between the lone pair on boron and the π bonds for carbon indicating that the molecule has some aromatic behaviour.
Pyridinium
Optimisation
A pyridinium ion was optimised using the B3LYP method with the 3-21G basis set followed by the 6-31G(d,p) basis set. The optimised .log file can be found at DOI:10042/23745 .
| File Name | AHall_pyr_opt_6_31 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 1 |
| Spin | Singlet |
| E(RB3LYP) | -248.66807392 a.u. |
| RMS Gradient Norm | 0.00004765 a.u. |
| Imaginary Freq | |
| Dipole Moment | 1.8719 Debye |
| Point Group | C2V |
| Job cpu time: | 0 days 0 hours 1 minutes 13.7 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000086 0.000450 YES
RMS Force 0.000028 0.000300 YES
Maximum Displacement 0.000672 0.001800 YES
RMS Displacement 0.000207 0.001200 YES
Predicted change in Energy=-1.043954D-07
Optimization completed.
-- Stationary point found.
Frequency Analysis

As the low frequencies are low compared to the 'real' frequencies, and non of the 'real' frequencies are negative, the optimisation has completed. The .log file can be found at DOI:10042/23970 and DOI:10042/23962 .
Low frequencies --- -6.9726 -0.0004 -0.0003 0.0006 17.3253 18.4293 Low frequencies --- 392.5033 404.0211 620.5147
MOs
Energy calculation was performed on the optimised pyridinium ion. The output files can be found at DOI:10042/23983
The opposite trend to that for boron is observed, as the nitrogen is more electrophilic than carbon and so has lower energy atomic orbitals. As the nitrogen orbitals are lower in energy they contribute more to the bonding orbitals than to the anti-bonding orbitals. This can be seen in the MOs below, as the bonding interactions in MOs 17 and 24 have much larger contributions from nitrogen than the anti-bonding interactions in MOs 18, 20, 22 and 25.
MO 16 |
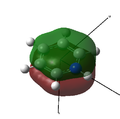
MO 17 |

MO 18 |
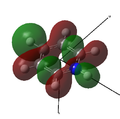
MO 19 |
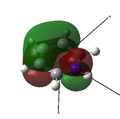
MO 20 |
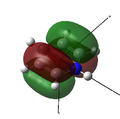
MO 21 (LUMO) |
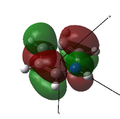
MO 22 (HOMO) |

MO 23 |

MO 24 |
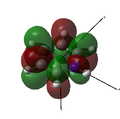
MO 25 |
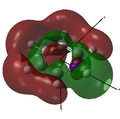
MO 26 |
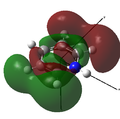
MO 27 |
NBO analysis

The NBO analysis of pyridinium indicates more similarities with benzene than boratabenezene, as the C-C bonds contain roughly equal contributions from both atoms and are sp2 with 33% 's' and 67% 'p'. The C-C π bonds are entirely 'p' based and contain equal contributions from each carbon atom. The C-H bonds are 65% carbon and 35% hydrogen with the carbon 31% 's' and 69% 's'.
The C-N bond is 37% C and 63% N, with carbon using 28% 's' and 72% 'p' and nitrogen using 37% 's' and 63% 'p'. C-N π bonds are also observed with 28% carbon contribution and 71% nitrogen with entirely 'p' orbitals. The N-H bond is 25% hydrogen and 75% nitrogen (27% 's', 73% 'p').
The core orbitals of carbon and nitrogen are entirely 's' based.
Significant mixing between the C-C and C-N π bonds is observed, implying that the system is aromatic.
Borazine
Optimisation
A molecule of borazine was optimised using the B3LYP method with the 3-21G basis set followed by the 6-31G(d,p) basis set. The .log files can be found at DOI:10042/23974 and DOI:10042/23975 .
| File Name | AHall_borz_opt_6_31 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -242.68458200 a.u. |
| RMS Gradient Norm | 0.00010596 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.0000 Debye |
| Point Group | D3H |
| Job cpu time: | 0 days 0 hours 0 minutes 50.0 seconds. |
Item Value Threshold Converged?
Maximum Force 0.000110 0.000450 YES
RMS Force 0.000047 0.000300 YES
Maximum Displacement 0.000527 0.001800 YES
RMS Displacement 0.000204 0.001200 YES
Predicted change in Energy=-3.565285D-07
Optimization completed.
-- Stationary point found.
Frequency Analysis

All of the low frequencies are much lower than the 'real' frequencies, and none of the 'real' frequencies are negative, indicating that the optimisation was successful. The .log file for the frequency analysis can be found at DOI:10042/23978 .
Low frequencies --- -14.3764 -14.0440 -10.4678 -0.0163 -0.0114 0.0336 Low frequencies --- 289.0878 289.0895 404.1828
MOs
Energy calculations were performed on the optimised borazine. The output files can be found at DOI:10042/23982 .

MO 16 |

MO 17 |

MO 18 |

MO 19 |

MO 20 |

MO 21 (LUMO) |

MO 22 (HOMO) |

MO 23 |

MO 24 |

MO 25 |

MO 26 |

MO 27 |
NBO analysis

The framework in borazine is made up from B-N bonds which are made up of 76% nitrogen (s( 38.56%), p( 61.43%)) and 24% boron (s( 31.24%), p( 68.51%)). The π bonds are 88% N and 22% B and are entirely 'p' based.
The B-H bonds are 45% boron (s( 37.48%),p( 62.45%)) and 55% hydrogen. The N-H bonds are 71% nitrogen (s(22.81%), p( 77.16%)) and 29% hydrogen.
The core orbitals on boron and nitrogen are entirely 's' based.
Mixing is observed between the B-N π framework, indicating that the molecule is aromatic.
Comparison
Charge Distribution
As expected the charge on benzene is very low, and all carbon atoms are identical, as are all hydrogen atoms. The charge on borazine - also relatively symmetrical - is much higher. Due to the symmetry there is no overall dipole, however due to the differences in electronegativity the boron atoms are very strongly positively charged, and the nitrogen atoms negatively charged. The charge on boron/nitrogen induces a smaller opposing charge on the hydrogen atoms which are attached to them.
Both boratabenzene and pyridinium have unsymmetrical charge on the carbon atoms, with those located closest to the heteroatom having greater charge. The boron atom has a positive charge which induces a more negative charge onto the neighboring carbon and hydrogen atoms. For the carbon atoms which are already partially negatively charged this reinforces the charge, making the carbon atoms more negative; however for hydrogen which is normally positively charged this opposes the charge, making the hydrogen atom less strongly charged. As the nitrogen atom in pyridinium has a negative charge this causes the opposite effect to boron, with the neighboring carbon atoms having an small induced positive charge and the neighboring hydrogen atom having its positive charge reinforced.
The charges in pyridinium are greater than in boratabenzene as the difference in electronegativities between carbon and nitrogen are greater than the difference between carbon and boron. The largest charge difference is for borazine, as this molecule contains alternative electropositive and electronegative atoms. The smallest charge difference is for benzene as there is no difference in electronegativities between the ring atoms, and the difference in electronegativities between carbon and hydrogen are small.
MOs
MO 17

| Benzene | Boratabenzene | Pyridinium | Borazine |
|---|---|---|---|
 |
 |
 |

|
The MOs for benzene and borazine are symmetrical, whereas those for boratabenzene and pyridinium are asymmetric due to the presence of the heteroatom. The nitrogen orbital contributes more to the MO as nitrogen is more electronegative than carbon, and so has atomic orbitals which are lower in energy, and so contribute more to the bonding orbitals than to the anti-bonding orbitals. The opposite is true for boron as boron is less electronegative than carbon and so has orbitals higher in energy which contribute less to bonding orbitals and more to anti-bonding orbitals.
MO 18/19
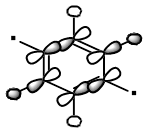
| Benzene | Boratabenzene | Pyridinium | Borazine | |
|---|---|---|---|---|
| MO 18 |  |
 |
 |

|
| MO 19 |  |
 |
 |

|
As this MO contains significant anti-bonding character the opposite effect to MO 17 can be observed, with boron contributing more to the MO than nitrogen due to its lower electronegativity. MOs 18 and 19 are degenerate for benzene and borazine, however for boratabenzene and pyridinium there is a significant difference in energy (0.00789 au. for borazine and 0.00296 au. for pyridine). This difference in energy is due to the asymmetry that the heteroatom induces. For pyridinium the higher energy MO has the same shape as the MO 19 for benzene, whereas for boratabenzene the MO with this shape is of lower energy.
MO 20/21

| Benzene | Boratabenzene | Pyridinium | Borazine | |
|---|---|---|---|---|
| MO 20 |  |
 |
 |

|
| MO 21 |  |
 |
 |

|
As with MOs 18 and 19, MOs 20 and 21 are degenerate for benzene and borazine, but not for boratabenzene and pyridinium. As the MOs with the same shape as MO 20 on benzene do not include the heteroatom for boratabenzene and pyridinium, these MOs are largely undistorted, however for borazine the contribution from nitrogen is significantly bigger than the contribution from boron as the orbital is bonding and so electronegative atoms contribute more than electropositive atoms. As was the case for MOs 18 and 19 the ordering is different for boratabenzene and pyridinium, probably due to the increased antibonding effects in MO 21 of boratabenzene compared to MO 20 of pyridinium due to the less electronegative boron atom contributing more.
The MOs with the same shape as MO 21 in benzene are much more varied, with the boron lobe in the boratabenzene being much larger than the nitrogen lobe in pyridinium due to the antibonding interactions in the orbital favouring the less electronegative atom. MO 21 for borazine is particularly interesting as the orbital is unsymmetrical due to the lack of a xz plane of symmetry meaning that there are two nitrogen atoms and one boron forming half of the MO, and the other half is formed of two borons and one nitrogen atom.
Effects on full MO diagram
Substituting a single boron or nitrogen atom into the ring causes a loss of symmetry, and therefore a loss of degeneracy between orbitals. This means that the MO diagrams for boratabenzene and pyridinium would not have as much degeneracy as for benzene, however as the difference in energy of the orbitals due to the loss of symmetry is low, the previously degenerate orbitals would still be close together. For borazine there is less loss of symmetry and so there would be more degenerate orbitals on the MO diagram.







































