Rep:Mod:AFJ1357
Introduction
Potential Energy Surfaces (PES)
What are they?
A PES shows the energy of a system as a function of the molecular geometry, by considering the internuclear repulsion, but ignoring the kinetic energy of the nuclei.
Maxima, Minima and Transition States
Maxima and minima are found on the PES where the gradient is equal to zero. To find out whether or not you have a maximum or a minimum you must perform a secondary differentiation. At a maximum the secondary derivative will be less than zero and at the minimum it will be greater than zero. The transition state is the highest energy point on the reaction path that requires the lowest energy to go to completion, and in the case of a 1D graph it is at the highest maximum.
Experimental Aims
In this computational experiment the reaction pathways of several different Diels-Alder reactions were studied, with the reactants and products being optimised to a minimum and the transition states being optimised to a maximum. A Diels-Alder reaction is also known as a [4+2] Cycloaddition, between a delocalised 2π system dienophile and a delocalised 4π system conjugated diene. A cheletropic reaction is also studied, which is another type of Cycloaddition, however in the case of a cheletropic reaction the two new bonds being formed are attached to a single atom.
By finding the energies of the reactants, products and transition states the energy barriers to reaction and the reaction energies can be calculated. From these values it can be determined whether the endo, exo or cheletropic reaction routes are kinetically or thermodynamically most favourable.
The calculations were first run using the PM6 method. This method is semi-empirical and returns geometries of the structures, however they may not be fully optimised to a minimum. To optimise the structures further the B3LYP DFT method is used, which often takes longer to run as it does not use as many assumptions as the PM6 method.
Nf710 (talk) 08:59, 18 November 2016 (UTC) Good understanding of the TS optimization, it would have been good if you could have gone into more detail about the methods here.
Exercise 1: Butadiene and Ethene
Molecular Orbital Diagram for the formation of Cyclohexene from Butadiene and Ethene

In order for the molecular orbitals to interact, they must share the same symmetry. This means that ungerade-ungerade interactions, or gerade-gerade interactions, are permitted but ungerade-gerade interactions are not. Therefore the molecular orbitals involved in a reaction must be of the same symmetry for that reaction to be 'allowed' and if they are of different symmetry the reaction is 'forbidden'.
For the gerade-gerade interaction and the ungerade-ungerade interaction the orbital overlap integral is non-zero, but for the ungerade-gerade interaction the orbital overlap integral is zero.
Visualising the Orbitals
Butadiene
| LUMO | HOMO |
|---|---|
 |

|
In the molecular orbitals above it can be seen that the LUMO of Butadiene is symmetrical and the HOMO of Butadiene is antisymmetrical.
Ethene
| LUMO | HOMO |
|---|---|
 |

|
In the molecular orbitals of Ethene the HOMO is symmetrical and the LUMO is antisymmetrical, the opposite of Butadiene.
Transition State
| Highest energy orbital in MO diagram | LUMO | HOMO | Lowest energy orbital in MO diagram |
|---|---|---|---|
 |
 |
 |

|
09:02, 18 November 2016 (UTC) Your reactant was the wrong conformer. But it looks correct in your TS.
Carbon - Carbon bond lengths throughout the reaction
| Bond (between numbered carbons above) | Reactants | Transition State | Products |
|---|---|---|---|
| 1 to 2 | 1.458 | 1.411 | 1.338 |
| 2 to 3 | 1.340 | 1.380 | 1.500 |
| 3 to 4 | - | 2.116 | 1.540 |
| 4 to 5 | 1.331 | 1.382 | 1.541 |
| 5 to 6 | - | 2.116 | 1.540 |
| 6 to 1 | 1.340 | 1.380 | 1.500 |
As the reaction progresses, the bonds becoming single bonds from double bonds (2-3, 4-5 and 6-1) increase in length, and the bond becoming a double bond from a single bond (1-2) decreases in length. This makes sense when compared to literature, as a typical sp3 - sp3 carbon single bond is reported as 1.544 Å, a typical sp2 - sp2 carbon single bond is reported as 1.46 Å and a typical sp2 - sp2 carbon double bond is reported as 1.334 Å.[1] These values are very similar to those in the table above, indicating the computational calculations were accurate. The partly formed carbon - carbon bonds in the TS (3-4 and 5-6) are between the lengths of a single bond and a double bond, as they has characteristics of both a single bond and carbon bond.
The reported value for the Van der Waals radius of carbon is 1.7 Å.[2] The two forming bonds in the transition state are shorter than twice the Van der Waals radius and therefore the two carbons have started to interact and the bonds have begun to form.
Vibrations
Vibration |
From the vibration above it can be seen that the formation of the two bonds is synchronus, which is expected as a Diels-Alder reaction is a type of concerted pericyclic reaction. The first positive vibration shows the ethene molecule rotating about the axis of the new bonds being formed.
Exercise 2:Benzoquinone and Cyclopentadiene
Molecular Orbitals
The HOMO of Butadiene and LUMO of Ethene are both antisymmetric and so the reaction is allowed. However, the opposite case is also true as the LUMO of Butadiene and HOMO of Ethene are both symmetric, so this reaction is also allowed. This, combined with the fact they have similar energies, means it is not a normal Diels-Alder reaction but an inverse demand Diels-Alder reaction. In the case of Benzoquinione and Cyclopentadiene neither the symmetry nor the relative energies are correct for the reaction to be an inverse demand Diels-Alder, so the reactions in this exercise are normal Diels-Alder reactions.
Endo Product
| Highest energy orbital in MO diagram | LUMO | HOMO | Lowest energy orbital in MO diagram |
|---|---|---|---|
 |
 |
 |

|
Exo Product
| Highest energy orbital in MO diagram | LUMO | HOMO | Lowest energy orbital in MO diagram |
|---|---|---|---|
 |
 |
 |

|
(It's quite difficult to see straight away what's going on in these diagrams in terms of symmetry. Best to choose an angle perpendicular to the reaction site to show the symmetry Tam10 (talk) 13:31, 10 November 2016 (UTC))
Energies associated with the Reaction
| Reactants | Transition State | Products | Barrier Energy | Reaction Energy | |
|---|---|---|---|---|---|
| Endo | -1510762.122 | -1510670.311 | -1510783.725 | 91.811 | -21.603 |
| Exo | -1510766.654 | -1510663.622 | -1510788.561 | 103.032 | -21.907 |
The endo product is the kinetically more favourable as it has the lower energy reaction barrier, and will therefore react faster. The exo product is the thermodynamically more favourable as it has the larger reaction energy, and is therefore the more thermodynamically stable of the two products.
Secondary Orbital Interactions
In the transition state of the endo product there is overlap between non-bonding p-orbitals of both of the reactants. This means there are secondary orbitals interactions, making the reaction more favourable, causing the reaction barrier energy to be lowered and the reaction rate to increase.
Nf710 (talk) 09:15, 18 November 2016 (UTC) Your endo is calculated correctly but you have got a slightly incorrect energy for the exo, This is a shame, because you have done this exercise well and concisely, you could have used Jmols and that would have made it easier to see the orbitals.
Exercise 3:Xylylene and SO2
IRC Files
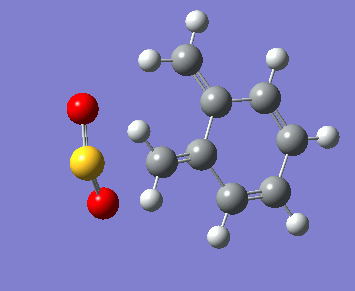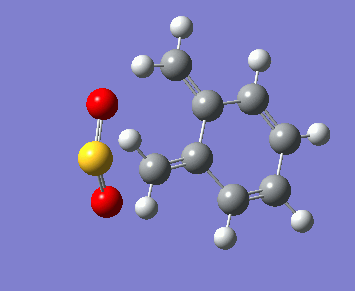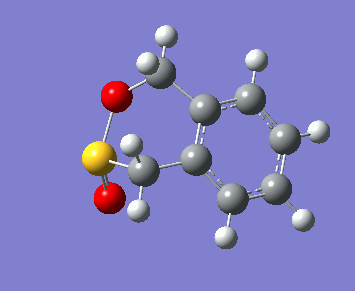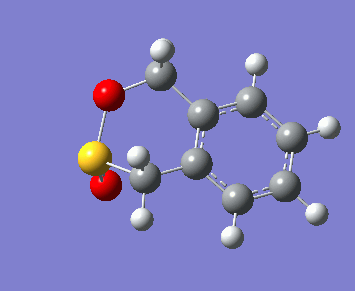
Below, the reaction paths of the three reaction routes can be seen.
| Endo Diels-Alder | Exo Diels-Alder | Cheletropic |
|---|---|---|
 |
 |
|
In the reaction paths the two molecules can be seen approaching each other and forming two new bonds. In the Diels-Alder reactions these bonds are a carbon-oxygen and a carbon-sulphur bond, forming a new 6-membered ring. In the Cheletropic reaction both new bonds formed are between a carbon and the single sulphur atom, forming a new 5-membered ring.
Energies associated with the Reactions
| Reactants | Transition State | Products | Barrier Energy | Reaction Energy | |
|---|---|---|---|---|---|
| Endo Diels-Alder | 178.390 | 237.763 | 56.984 | 59.373 | -121.406 |
| Exo Diels-Alder | 177.686 | 241.746 | 56.855 | 64.060 | -121.831 |
| Cheletropic | 177.607 | 260.085 | -0.005 | 82.477 | -177.612 |

From the data above it can be seen that the endo Diels-Alder reaction is the faster, kinetically favoured reaction, due to the energy barrier to reaction being the lowest of the three routes. It can also be seen that the cheletropic reaction route is most the thermodynamically stable, as it has the largest reaction energy of three routes, with the product having the lowest energy. This may be due to it having a more stable,less strained and more planar 5-membered ring in the structure, compared with the slightly bent structure of the Diels-Alder products.
(Yes, this is probably the reason for the stability of the cheletropic product. It's probably also why it has the highest barrier - the previously sp2 are heavily distorted and strained in the TS. This is visible in the IRCs that you've provided Tam10 (talk) 13:31, 10 November 2016 (UTC))
To the left is a diagram showing the reaction profiles of the three reactions, having set the energies of the reactants at infinite separation to be zero.
Aromaticity
Throughout the reaction the bonding present in the 6-membered ring changes. In the original Xylylene molecule it is a conjugated diene system, however in the final o-Xylylene-SO2 molecule the bonding has become aromatic. 6-membered aromatic systems are very thermodynamically stable and therefore highly favourable. This aromaticity is the driving force behind the reaction and explains the high instability of the Xylyene molecule.