Rep:Mod:AER1116
Conformational Analysis using Molecular Mechanics
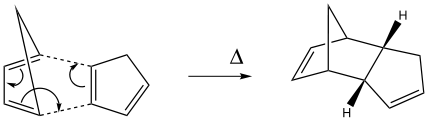
The Hydrogenation of Cyclopentadiene Dimer
The geometries and energies of the exo and endo dimers of cyclopentadiene were examined using the MMFF94s force field. The main differences between the two come in the angle bending and electrostatic energies. In both of these the value for the endo form is greater i.e. it is less stable. This is not surprising as there appears to be clear clashing between the two hydrogen atoms at the ring junction and the hydrogens of the CH2 group sticking out of the double ring.
The endo dimer is the product favoured by the reaction, despite having a higher total energy as shown in the calculations below. This suggests that the dimerisation is under kinetic control and that the endo formation must have the lowest energy transition state for it to be the preferred conformer. The formation of the endo-cyclopentadiene dimer is an example of a bispericyclic reaction[1] and its transition state is shown below.
| Structure | Total Energy | Bond Stretching Energy | Angle Bending Energy | Stretch Bending Energy | Torsional Energy | Out of Plane Bending Energy | Van der Waals Energy | Electrostatic Energy |
|---|---|---|---|---|---|---|---|---|
| Exo | 55.37 | 3.54 | 30.77 | -2.04 | -2.73 | 0.01 | 12.80 | 13.01 |
| Endo | 58.19 | 3.47 | 33.18 | -2.08 | -2.95 | 0.02 | 12.36 | 14.18 |
Exo-Cyclopentadiene Endo-Cyclopentadiene
Analysis was then conducted on the two possible hydrogenation products from the endo dimer, one for each double bond. The data obtained suggests that the double bond in the bent ring is significantly easier to hydrogenate than that of the flat ring, at least, from a thermodynamic point of view. This supports the obervation that product 1 is only formed after a lengthy reaction time and also suggests that the reaction may be under thermodynamic control.
The biggest contributions to the overall energy are from angle bending and Van der Waals' interactions. Unsuprisingly these are also where there are the biggest differences between the two with product 2 having a lower value for both. Angle bending is the biggest factor contributing roughly 60% of the total energy with Van der Waals' providing nearly 25%.
| Structure | Total Energy | Bond Stretching Energy | Angle Bending Energy | Stretch Bending Energy | Torsional Energy | Out of Plane Bending Energy | Van der Waals Energy | Electrostatic Energy |
|---|---|---|---|---|---|---|---|---|
| 1 | 50.45 | 3.31 | 31.94 | -2.10 | -1.47 | 0.01 | 13.64 | 5.12 |
| 2 | 41.26 | 2.82 | 24.68 | -1.66 | -0.36 | 0.00 | 10.63 | 5.15 |
Hydrogenation Product 1 Hydrogenation Product 2
Two isomers of an intermediate from the preparation of Taxol, one with the carbonyl group sticking up and one with it down, were modelled using MMFF94s. The isomers can be separated as the the complex, strained ring system limits rotation about the sp2-sp3 bond [2]. The results show that isomer 10, the one where the carbonyl group points down (opposite direction to the two hydrogen atoms at the cyclohexane ring junction) is the most stable, so may be preferred by the equilibrium. This is due to the fact that when pointing down the carbonyl group creates a chair type structure whereas when pointing down a less stable, twist boat is formed [3].
The taxol intermediates are formed by an oxy-cope [3,3] sigmatropic rearrangement. The keto form shown in intermediates 9 and 10 has been shown to be so much more thermodynamically favourable over the enol forms that the tautomerisation is essentially irreversible [3]. This explains why the alkene is so slow to react which would break this favourable arrangement.
Interesting, if the two fixed hydrogen atoms on the cyclohexane ring are moved into the downward position in intermediate 10, the energy is raised to 136.4 kJmol-1 . So despite breaking the ideal chair orientation it's still more stable than the twist boat in species 9. By tweaking the structure of the ring and using different levels of twist and bend a conformation with an energy as low as 70.9 kcalmol-1 can be obtained. This is probably due to the fact that every cyclohexane chair has two possible arrangements, this lower energy conformation probably invokes a more stable, preferential form to the one first examined.
Lowest Energy Taxol Intermediate
| Structure | Total Energy | Bond Stretching Energy | Angle Bending Energy | Stretch Bending Energy | Torsional Energy | Out of Plane Bending Energy | Van der Waals Energy | Electrostatic Energy |
|---|---|---|---|---|---|---|---|---|
| 9 | 144.46 | 13.61 | 67.29 | 0.01 | 8.24 | 2.37 | 51.01 | 1.92 |
| 10 | 130.98 | 14.23 | 57.39 | 0.33 | 5.74 | 2.39 | 49.52 | 1.39 |
Taxol Intermediate 9 Taxol Intermediate 10
Spectroscopic Simulation using Quantum Mechanics
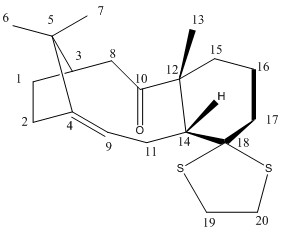
The carbon and hydrogen NMR spectra of a taxol intermediate (18) were simulated using Gaussian and the MMFF94s force field. The results could then be compared with the literature value.
| NMR | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Literature Value[4] | 211.49 | 148.72 | 120.90 | 74.61 | 60.53 | 51.30 | 50.94 | 45.53 | 43.28 | 40.82 | 38.73 | 36.79 | 35.47 | 30.84 | 30.00 | 25.56 | 25.35 | 22.21 | 21.39 | 19.83 |
| Simulated Value[5] | 212.94 | 147.91 | 120.12 | 93.17 | 65.83 | 54.92 | 54.92 | 49.57 | 48.01 | 45.69 | 44.05 | 41.24 | 38.63 | 33.62 | 32.47 | 28.31 | 26.41 | 24.47 | 24.07 | 22.48 |
| Deviation | 1.45 | 0.81 | 0.78 | 18.56 | 5.30 | 3.62 | 3.98 | 4.04 | 4.73 | 4.87 | 5.32 | 4.45 | 3.16 | 2.78 | 2.47 | 2.75 | 1.06 | 2.26 | 2.68 | 2.65 |
| Assignment | 10 | 4 | 9 | 18 | 14 | 12 | 3 | 5 | 17 | 20 | 19 | 15 | 8 | 13 | 11 | 2 | 6 | 1 | 16 | 7 |
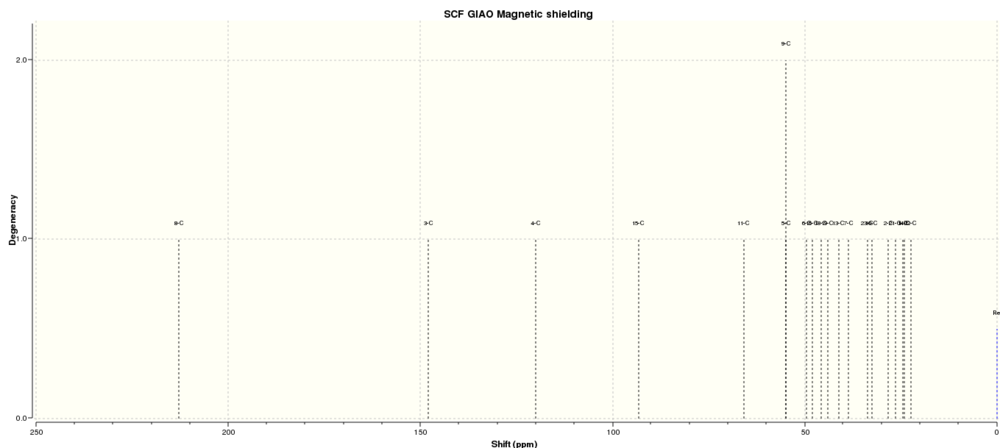
The two spectra show a reasonably good agreement with the main disparity being in the couple of peaks between 65-100 ppm. There is a small difference between all values, but considering the limitations of the simulation and the fact that the literature spectra are over 20 years old the agreement is very good. Interestingly, the simulation gives two carbon atoms in different environments the same chemical shift. This appears to simply be a quirk in its method of calculation.
| NMR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Literature Value [4] | 5.21 (1H,m) | 3.00-2.70 (6H,m) | 2.70-2.35 (4H,m) | 2.20-1.70 (6H,m) | 1.58 (1H,t) | 1.50-1.20 (3H,m) | 1.10 (3H,s) | 1.07 (3H,s) | 1.03 (3H, s) | |
| Simulated Value[5] | 6.00 (1H,m) | 3.07, 3.03, 2.88 (3x2H,m) | 2.56 (1H,t) | 2.31, 2.21, 2.17(3x2H,m) | 2.07, 1.89 (2x2H,m) | 2.00, (1H,m) | 1.43 (3H, s) | 1.38 (2H, m) | 1.28 (3H, s) | 1.15 (3H,s) |
| Assignment | 7 | 15, 14, 8 | 10 | 2, 6. 1 | 13, 12 | 3 | 9 | 11 | 5 | 4 |

The hydrogen NMR spectra show a larger disparity. This may well be due to the very flexible nature of the multiple ring system causing a large number of fluxional species. However, overall the agreement is decent and a clear comparison can be made. Perhaps due to the age of the literature spectra, it groups peaks together if they appear at very similar shifts (e.g. the 3.00-2.70 6H assignments) whereas the simulated spectra gives a view of them individually. The simulated spectra appears to have calculated a higher shift for the vast majority of the peaks, compared to the literature. Sometimes this difference can be as high as 1 ppm. As hydrogen NMR spectra are spread over a smaller range than carbon and, in a large molecule like the taxol intermediate, there is more opportunity for overlap between peaks it is perhaps unsurprising that the spectra are not as similar as in the carbon comparison. Furthermore, in the simulated spectra all hydrogen atoms, were presented individually and those in equivalent groups e.g. methyl or -CH2- had to be averaged out manually.
The entropy and vibrational calculation gives the free energy (sum of zero point and thermal energies) to be -1651.4 Hartrees[5]. As this is a large, negative value it corroborates the earlier findings that when the carbonyl group is pointing down, it gives a more favourable isomer than when pointing up.
Analysis of the Properties of Alkene Epoxides
Crystal Structure
Shi Catalyst
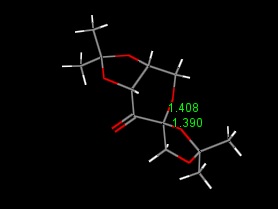
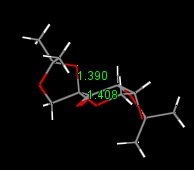
The crystal structure of an intermediate of the Shi catalyst was examined with particular attention towards the anomeric centre. This is located on the six membered ring, which has a sugar structure. The two C-O bonds lengths were calculated and it was shown that the C-O bond within the ring was longer than that outside the ring. This may well be a manifestation of the anomeric effect as this oxygen atom is in the preferred axial position, alpha- to another heteroatom so may have a slightly stronger bond to the carbon of the ring. This extra strength is shown as a shortening of the bond.
Jacobsen Catalyst
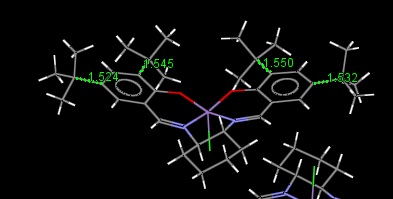
Analysis of the crystal structure of the intermediate of the Jacobsen catalyst shows that there are two different bond lengths between the carbon atoms of the phenyl rings and the tert-butyl groups. Two of these bulky groups, one from each ring, are quite close together over the less occupied side of the catalyst opposite the cyclohexane ring. The bonds between these groups to the phenyl groups are longer. This could be due to some sort of steric interaction between the branches of these two groups. This longer bond could also be to prevent clashing with the other tert-butyl group on the same ring. The other two tert-butyl groups are pointing away from the centre of the catalyst and don't directly hinder any other groups. These have shorter bonds to the phenyl ring.
NMR Analysis
S- Styrene Oxide
| NMR | |||||
|---|---|---|---|---|---|
| Literature[6] | 2.81 (dd, J = 2.6, 5.5 Hz, 1H) | 3.15(dd, J = 4.1, 5.5 Hz, 1H) | 3.86 (dd, J = 2.6, 4.0 Hz, 1H) | 7.37-7.25 (m, 5H) | |
| Simulated[7] [8] | 2.54 (dd, J = 1.9, 5.6, 1H) | 3.12 (dd, J = 4.4, 5.6, 1H) | 3.66 (dd, J = 0.8, 4.1, 1H) | 7.30 (m, 1H) | 7.49 (m, 4H) |
| Assigment | 2 | 1 | 3 | 4 | 5,6,7,8 |
| NMR | ||||||||
|---|---|---|---|---|---|---|---|---|
| Literature[9] | 51.2 (1C) | 52.3 (1C) | 125.5 (1C) | 128.2 (1C) | 128.3 (1C) | 128.5 (1C) | 128.6 (1C) | 137.6 (1C) |
| Simulated[7] | 53.5 (1C) | 54.1 (1C) | 118.3 (1C) | 123.0 (2C) | 123.4 (1C) | 124.1 (1C) | 135.1 (1C) | |
| Assigment | 1 | 2 | 4 | 6,8 | 7 | 5 | 3 |
R,R-β-methylstyrene oxide
| NMR | ||||
|---|---|---|---|---|
| Literature [10] | 1.07 (d, J = 5.4 Hz, 3 H) | 3.33 (dq, J = 5.4, 4.3 Hz, 1 H) | 4.05 (d, J = 4.3 Hz, 1 H) | 7.25-7.36 (m, 5 H) |
| Simulated[11] [12] | 0.99(d, J = 5.8 Hz , 3H) | 3.28 (dq, 4.7 Hz, 1H) | 3.99 (d, J = 4.7 Hz 1H) | 7.39-7.53 (m, 5H) |
| Assignment | 2 | 1 | 3 | 4-7 |
| NMR | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Literature[10] | 12.5 | 55.1 | 57.5 | 126.5 | 127.4 | 128.0 | 135.5 | ||
| Simulated[11] | 13.1 | 57.7 | 59.0 | 121.4 | 122.4 | 122.7 | 123.1 | 123.4 | 132.8 |
| Assignment | 1 | 2 | 3 | 7 | 9 | 4 | 8 | 6 | 4 |
All the NMR shifts were reasonably close to the values found in the literature. There were greater disparities with the coupling constants, although these were still fairly similar. The coupling constants are very dependent on the exact structural configuration used, there may also have been the possibility of longer range coupling in the phenyl ring, which may not have been accurately simulated.
As expected, the NMR spectra show no difference between the two enantiomers for each epoxide. A chiral agent must be added for NMR to show the different species. Hence, only one enantiomer per epoxide is presented above. Therefore, to analyse the different enantiomers, both in this report and in the upcoming experiment, calculation of the optical rotation must be used.
Optical Rotation
Literature Values
R-Styrene Oxide = + 5.05o [13]
S-Styrene Oxide = -24.1o [14]
R,R-β-methylstyrene oxide - No literature value found
S,S-β-methylstyrene oxide = -41.7o [15]
Simulated Values
R-Styrene Oxide = + 30.56 o [16]
S-Styrene Oxide = - 30.59 o [17]
R,R-β-methylstyrene oxide = + 37.39 o [18]
S,S-β-methylstyrene oxide = -37.59 o [19]
Most of the literature values are fairly similar to those reported in literature. The exception is for R-Styrene oxide where there is a difference of approximately 25 o, over 80% of the simulated value. An explanation for this is that the only literature available was based on an experiment conducted in 1965, so may have used equipment or techniques now considered outdated. The simulated values will be useful for the upcoming experiment where these epoxides will actually be synthesised. The optical rotation will be used to determine the selectivity of the Shi and Jacobsen catalysts.
N.B. No literature value for R,R-β-methylstyrene oxide was found, but this can be expected to have the same magnitude as that of S,S although in the opposite direction.
Analysis of Transition State Energies
The energies of the provided transition states of the epoxidations were compared. The energy difference can be used to give the equilibrium constant K. This gives the ratio of the amounts of the two isomers which can be, in turn, used to calculate the enantiomeric excess. The following equations are used in this calculation:
where K is the equilibrium constant giving the relative amounts of R and S
Where R and S are the respective amounts of the two enantiomers
E.g. for the first calculation kJmol-1 so rearranging the equation gives . So every mole of styrene gives 0.298mol R and 0.702mol S. Using the expression for enatiomeric excess
N.B. The Gaussian log file presents energies in Hartrees. These were converted to kJmol-1 before calculation.
| Shi Epoxidation of Styrene | ||||
|---|---|---|---|---|
| R enatiomer (kJmol-1) | S Enantiomer (kJmol-1) | Free Energy Difference(kJmol-1) | K | ee |
| -3422741 | -3422744 | 3 | 0.298 | 40.4% ee S |
| -3422737 | -3422723 | 14 | 0.00352 | 99.3% ee R |
| -3422755 | -3422735 | 20 | 3.12x10-4 | 99.9% ee R |
| -3422760 | -3422757 | 3 | 0.298 | 40.4 ee R |
The reported literature value is 81% ee R- Styrene oxide[20].This suggests that either the second or third transition states modelled here are closest to that actually formed in the reaction.
| Jacobsen Epoxidation of Styrene | ||||
|---|---|---|---|---|
| R enatiomer (kJmol-1) | S Enantiomer (kJmol-1) | Free Energy Difference(kJmol-1) | K | ee |
| -8779274 | -8779294 | 20 | 3.12x10-4 | 99.9% ee S |
| -8779278 | -8779280 | 2 | 0.446 | 10.8% ee S |
The literature gives an enatiomeric excess of 36% R[21]. This doesn't correspond to either of the calculated values and further analysis of these transition states may be needed.
Some of the calculated values give e.e. values of 99% or similar. These are very unlikely to be obtained in any form of experimental procedure.
NCI Surface
The NCI surface below was calculated for the first transition state of S styrene with the Shi catalyst. The analysis of this transition state gave an enantiomeric excess of 40% S in the earlier calculations. The surface shows a large. mildly attractive interaction around the overlap of styrene double bond (or where is was in the starting material) and the central part of the Shi catalyst around where the bond is formed. This large, favourable interaction may explain why this S enantiomer is favoured for this transition state. There is very little repulsive interaction, and these small patches only really appear within the structure of the Shi catalyst itself.


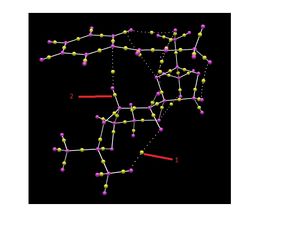
QTAIM Analysis
QTAIM analysis was also conducted on the first Shi transition state for S Styrene. This shows around 5 weak non-covalent BCPs between the styrene molecule and the catalyst. The flat styrene molecule forms an almost parallel orientation to the central part of the catalyst leaving plenty of hydrogen atoms available to interact with the oxygen atoms of the Shi. Interestingly, the model also predicts a weak non-covalent BCP between one of the central oxygen atoms and a methyl hydrogen of one of the end branches. Most of the BCPs between the styrene molecule and the catalyst appear to be slightly closer to the styrene molecule. This may be due to the electron rich, resonance stablised structure of styrene.
Candidate for Future Investigation
The epoxide 2,3-epoxy-2,3-dihydro-phenalen-1-one has a molecular weight of 196 and a reported optical rotation of -427.8 [22]. The given synthesis uses NaOCl as an oxidising agent and a quaternary ammonium salt catalyst to synthesise the epoxide from the enone. This method gives a reported 98% yield with 86% enatiomeric excess[22].
References
- ↑ 1.0 1.1 H.Rzepa,"Organic Pericyclic Reactions",1978-2014,DOI:2011-10-12T07:24:07Z
- ↑ P.Lloyd-Williams and E.Giralt,"Atropisomerism, biphenyls and the Suzuki coupling: peptide antibiotics",E. Chem. Soc.,2001,30,145.DOI:10.1039/B001971M
- ↑ 3.0 3.1 S.W.Elmore and L.A.Paquette,"The first thermally induced retro-oxy-cope rearrangement",Tetrahedron Lett.,1991,32,319-322.DOI:10.1016/S0040-4039(00)92617-0
- ↑ 4.0 4.1 L.Paquette, N.A.Pegg,D.Toops, G.D.Maynard, R.D, Rogers,"[3.3] Sigmatropy within 1-vinyl-2-alkenyl-7,7-dimethyl-exo-norbornan-2-ols. The first atropselective oxyanionic Cope rearrangement",J. Am.Chem. Soc.,'1990,112,277-283.DOI:10.1021/ja00157a043
- ↑ 5.0 5.1 5.2 DOI:10042/26958
- ↑ H.Toda, R.Imae, N.Itoh ,"Efficient biocatalysis for the production of enantiopure (S)-epoxides using a styrene monooxygenase (SMO) and Leifsonia alcohol dehydrogenase (LSADH) system",Tetrahedron-Assymetr,2012,23, 1542-1549 DOI:10.1016/tetasy.2012.09.017 ,
- ↑ 7.0 7.1 DOI:10042/27243
- ↑ DOI:10042/27255
- ↑ D.Xiong, X.Hu, S.Wang, C.X.Miao, C.X.W.Sun ,"Biaryl-Bridged Salalen Ligands and Their Application in Titanium-Catalyzed Asymmetric Epoxidation of Olefins with Aqueous H2O2",Eur J Org Chem,2011,23, 4289-4292DOI:10.1002/ejoc.201100512 ,
- ↑ 10.0 10.1 M.K.Tse, M.Klawonn, S.Bohr, C.Doebler, G.Anilkumar, H.Hugl, W.Maegerlein, M.Beller,"Convenient Method for Epoxidation of Alkenes Using Aqueous Hydrogen Peroxide",Org. Lett.,2005,7,987-990.DOI:10.1021/ol047604i
- ↑ 11.0 11.1 DOI:10042/27248
- ↑ DOI:10042/27250
- ↑ G.Berti, F.Bottari, P.L.Ferrarini, B.Macchia," Stereochemistry of the Additions of Acids to Stilbene and Styrene Oxides",J.Org.Chem.,1965,30,4091-4096.DOI:10.1021/jo01023a026
- ↑ H.C.Kolb, K.B.Sharpless"A simplified procedure for the stereospecific transformation of 1,2-diols into epoxides",Tetrahedron,1992,48,10515-10530.DOI:10.1016/S0040-4020(01)88439-6
- ↑ S.Koya, Y.Nishoika, H.Mizoguchi, T.Uchida, T.Katsuki,"Asymmetric Epoxidation of Conjugated Olefins with Dioxygen",Angew, Chem. Int.Edit.,2012,51,8243-8246.DOI:10.1002/anie.201201848
- ↑ DOI:10042/27231
- ↑ DOI:10042/27232
- ↑ DOI:10042/27233
- ↑ DOI:10042/27234
- ↑ H.Tian, X.She, J.Xu and Y.Shi,"Enantioselective Epoxidation of Terminal Olefins by Chiral Dioxirane",Org. Lett.,2001,3,1929-1931.DOI:10.1021/ol010066e
- ↑ B.Brandes and E.Jacobsen,"Synthesis of enantiopure 3-chlorostyrene oxide via an asymmetric epoxidation-hydrolytic kinetic resolution sequence",Tetrahedron Assymet.,1997,8,3927-3933.DOI:10.1016/S0957-4166(97)00568-5
- ↑ 22.0 22.1 22.2 B.Lygo, S.D.Gardiner, M.C.McLeod, D.C.M.To,"Diastereo- and enantioselective synthesis of α,β-epoxyketones using aqueous NaOCl in conjunction with dihydrocinchonidine derived phase-transfercatalysis at room temperature. Scope and limitations",Organic & Biomolecular Chemistry,2007,5,2283-2290.DOI:10.1039/b706546a