Rep:Mod:694859msyj
Introduction
Various organic structures and reactivity can be modelled using computational techniques. These modelling techniques are used to rationalise the outcomes of certain reactions as well as to predict the outcomes of new reactions. In this experiment, ChemBio3D, Avogadro, Gaussview and HPC are used for obtaining the structures as well as properties of the reactants, transition states, and products of the epoxidation reaction.
Conformational Analysis using Molecular Mechanics
In this part of the experiment, ChemDraw was used to construct the molecule (saved as .cdxml file) and then opened in Avogadro for analysis. The force field used was MMFF94s, and the algorithm is set to "Conjugate Gradient". In this calculation, the molecular geometry is optimised to a minimum energy and the bond length, angle strain, steric effects and Van der Waals contributions are calculated based on the final energy.
Limitations of this model
Being a parametric method built upon well-characterised and known molecules, this method is interpolative rather than extrapolative - the results obtained will not be too different from what we know, hence if the particular molecule is an anomaly, this model will not be able to depict this anomaly.
In addition, as the model tries to optimise the geometry of the molecule by obtaining the lowest energy conformation (and thus most thermodynamically stable), it is difficult to model molecules which react under "kinetic control". It is also difficult to take into account stereoelectronic effects, hyperconjugation, aromaticity, frontier orbital interactions and secondary orbital effects as well.
Hydrogenation of Cyclopentadiene Dimer
Optimising geometries of dimers 1 to 4
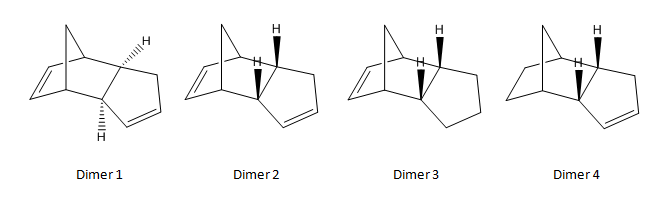
When cyclopentadiene dimerizes, it forms the endo product (Dimer 2) instead of the exo product (Dimer 1) due to increased stability of the endo product. Hydrogenation of the dimer product in the endo form produces a dihydro derivative - Dimer 3 or Dimer 4. Modelling is done to calculate the geometry and energies of all the four dimer species, and the results are as shown below.
The programme "Avogadro" is used for optimisation of the geometries of the dimers. The force field option was set to MMFF94(s) and the algorithm was set to "Conjugate Gradients".
| |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
55.37344 kcal/mol |
58.19070 kcal/mol |
50.44573 kcal/mol |
41.25749 kcal/mol |
| Property | Dimer 1 | Dimer 2 | Dimer 3 | Dimer 4 |
|---|---|---|---|---|
| Total bond stretching energy (kcal/mol) | 3.54305 | 3.46742 | 3.31118 | 2.82305 |
| Total angle bending energy (kcal/mol) | 30.77271 | 33.19129 | 31.93876 | 24.68554 |
| Total stretch bending energy (kcal/mol) | -2.04139 | -2.08214 | -2.10230 | -1.65715 |
| Total torsional energy (kcal/mol) | -2.73105 | -2.94948 | -1.47266 | -0.37809 |
| Total Out-of-plane bending energy (kcal/mol) | 0.01486 | 0.02195 | 0.01315 | 0.00028 |
| Total Van der Waals energy (kcal/mol) | 12.80155 | 12.35726 | 13.63808 | 10.63684 |
| Total electrostatic energy (kcal/mol) | 13.01372 | 14.18435 | 5.11951 | 5.14702 |
Discussion
Comparing dimers 1 & 2
Comparing dimers 1 (exo) and 2 (endo), it is shown that dimer 1 has a lower energy value than dimer 2 and hence it is more stable. However, it is mentioned that cyclopentadiene dimerizes to form dimer 2 (endo) instead.
As mentioned previously under limitations of this modelling method - it is difficult to model kinetically controlled reactions even if they might be more favoured due to stereoelectronic reasons for example. In the event where the transition state of the endo product is closer in energy to the starting material than the exo product, there is a tendency for the starting material to proceed via the lower energy transition state, and hence forming the endo product, which may be higher in energy than the exo product. This is an illustration of the Hammond's Postulate: The starting material, intermediate or product closest in energy to the transition state of interest will be the most similar in structure.
Another reason why the endo product (kinetic product) is formed is due to presence of secondary orbital overlap effect. Secondary orbital overlap is also present in the exo transition state but there is much less efficient overlap, hence resulting in the preferential formation of the endo transition state.
Thus, the Diels-Alder reaction of two cyclopentadiene molecules are under kinetic control.
Comparing dimers 3 & 4
Dimer 4 has a lower energy than dimer 3, hence indicating that dimer 4 is the thermodynamic product while dimer 3 is the kinetic product.
Dimer 3 has larger bond stretching, angle bending and Van der Waals energy (possibly due to increased steric hindrance due to presence of double bond in 5-membered ring), hence indicating that there are additional strains in the molecule as compared to dimer 4. Having a double bond in the five- or six-membered ring gives rise to difference in such values. Dimer 3 has the double bond in the six membered ring, leaving the cyclopentane all singly bonded (all sp3 hybridised). Although not very much significant, there is still some ring strain in the molecule. However, for dimer 4, the double bond is at the five-membered ring (carbons at the double bond are sp2 hybridised) and hence reducing some ring strain present in the molecule.
Optimisation of Intermediates 9 and 10
In the total synthesis of Taxol - a vital drug in treating ovarian cancers, Paquette[1] proposed two possible key intermediates - 9 and 10. The difference between these two intermediates are the positions of the carbonyl oxygen, either pointing upwards (Intermediate 9) or downwards (Intermediate 10).
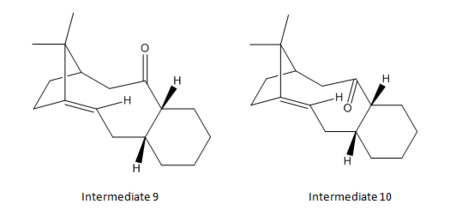
The programme "Avogadro" is used for optimisation of the geometries of the dimers. The force field option was set to MMFF94(s) and the algorithm was set to "Conjugate Gradients".
The results obtained are shown below:
| |
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
70.55643 kcal/mol |
60.55495 kcal/mol |
81.75698 kcal/mol |
71.51272 kcal/mol | |||||||||
Discussion
Comparison of energy values (and their stabilities) between parent and intermediates 9 & 10
The energy values of the parent molecules are computed to show the difference in the structures and stabilities. It is observed that the parent molecules (hydrogenation products) have a larger energy values than the intermediates, hence implying that the parent molecules will tend to be more reactive than the more stable intermediates of lower energy values. In general, intermediate and parent molecule 10 have lower energies than intermediate and parent molecule 9 respectively.
As Intermediate 10 is about 10kcal/mol less than intermediate 9, it can be concluded that intermediate 10 is the more stable than intermediate 9. One possible reason can be due to the large difference in angle bending energies by about 10kcal/mol for intermediate 9 (and parent 9) as compared to intermediate 10 (and parent 10). This angle bending effect cause deviations from the ideal angles, resulting in the different stabilities of the molecules. Hence, for a larger angle bending energy molecule, there will be a larger deviation from ideality, thus resulting in intermediate 9 being less stable than intermediate 10 (same for their parent molecules).
Conformations of the cyclohexane ring
Having a cyclohexane ring in the intermediate suggests the possibility of different conformations possible in the transition state - chair, boat, twist-boat and half chair. After several attempts at changing the conformations of the cyclohexane ring, it was found that the chair structure of the cyclohexane ring gives the lowest energy value, and hence is the most stable conformation as shown in the diagrams above.
Calculations were done with the cyclohexane being in the boat form, and the results are shown below:
| |
||||||
|---|---|---|---|---|---|---|
81.24416 kcal/mol |
70.79793 kcal/mol | |||||
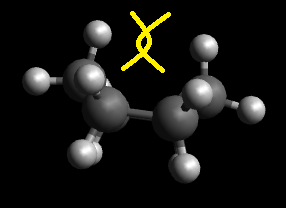
In the chair conformation, there is no torsional strain, with perfect sp3 bond angles. On the other hand, the boat conformation suffers from torsional strain and steric flagpole interactions between the hydrogens pointing towards each other (Figure 1), resulting in a much higher energy value for the boat conformation. This effect is also present in the twist-boat structure and more amplified in the half-chair structure. It is also good to note that ring flipping is not possible in the molecules - locked cyclohexane, as there is a long chain attached to the cyclohexane ring, hence unlikely to be able to flip easily.
Thus, the lowest energy conformation of the intermediates would be when the cyclohexane ring is in the chair conformation.
Atropisomerism
Atropisomers are stereoisomers formed via hindered rotation about single bonds as the barrier to rotation is so high for isolation of conformers to be possible[2]. Unlike other chiral compounds where isomerization occurs via chemical reactions, equilibration process for atropisomerism is usually controlled by thermal conditions.
In the two compounds 9 and 10, the only difference between them is the conformation of the six-membered ring - chair and boat atropisomers. Comparing the energy values of the chair and boat atropisomers, it is obvious that the chair conformations are in general of lower energies than the boat conformations. This is in agreement with literature[3], where hydrogenation of the compound of their interest at room temperature yields a six-membered chair, with the boat atropisomer being unstable.
Here, comparing the two intermediates with the same more stable chair conformation, it is shown that intermediate 10 of the chair conformation shows has a lower energy than intermediate 9, hence intermediate 10 is the more stable isomer.
Hyperstable alkenes
Olefin strain energy (OS) is the difference between the strain energy of an olefin and its parent hydrocarbon[4]. This energy difference is extremely useful in the interpretation and prediction of stability and reactivity of bridgehead olefins. Hyperstable olefins, unlike usual olefins, are in fact thermodynamically more stable and less strained than the parent hydrocarbon (showing negative OS values). Due to the location of the bridgehead location of the double bonds, hyperstable olefins have its reactivity significantly decreased.
This decreased reactivity is not caused by a stronger σ-bond or steric hindrance[5] of the molecule. Instead, the cage structure gives the alkene unique stability, coupled with the fact that there is a larger strain energy of the parent polycycloalkane.
The alkene used in this experiment (also seen in intermediate 9 and 10) are hyperstable alkenes, and hence they both have lower energies than their parent molecules 9 and 10 as shown in the table above.
Interesting literature data & possibility for further investigations
From previous literature[6], it was found that some cycloalkenes are less strained than the corresponding cycloalkanes. This increase in strain energy was suggested to be due to interactions of vicinal and transannular hydrogens. Due to their hyperstability, hyperstable olefins are reluctant to be hydrogenated even with forcing conditions(such as Pd-C or PtO2 as catalyst, 50 °C, 20 atm H2, several hours, different solvents), but it was found that they can be reduced with diimide or epoxidized with peroxycarboxylic acids or dimethyldioxirane.
Possibility for future research
Trisubstituted bridgehead olefins which are highly strained can be investigated for calculations of their stability. Instead of the small hydrogen or methyl groups at the bridgehead (in this experiment), more bulky groups (Phenyl, t-Butyl etc) can be used for further investigation to check for any stabilisation electronically.
Spectroscopic Simulation using Quantum Mechanics
Quantum mechanics can be used to predict NMR spectra. These results obtained from computational methods can be compared with the chemical shifts reported in previous literature to ensure accuracy and reliability of the simulations.
In this part of the experiment, both molecules 17 and 18 were used in the prediction of NMR spectra. Molecules 17 and 18 are derivatives of intermediates 9 and 10 respectively in the previous section.
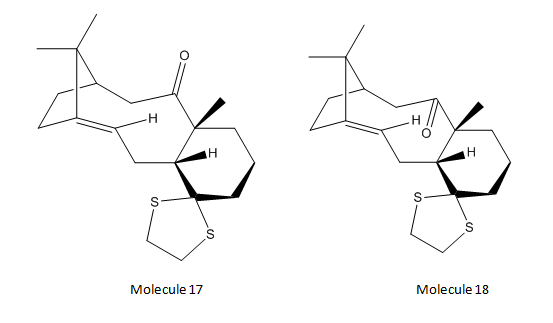
Molecules 17 and 18 were drawn in ChemBio3D before optimisation and minimisation of geometry using MMFF94s mechanics force field in Avogadro. The results obtained are shown below:
| Property | Molecule 17 | Molecule 18 | ||||
|---|---|---|---|---|---|---|
118.05374 kcal/mol |
100.50515 kcal/mol |
Under "Extensions", Gaussian was then selected. The calculation was set to "Geometry optimisation", B3LYP with basis set 6-31G(d,p). The keyword line was set to be: # B3LYP/6-31G(d,p) Opt SCRF=(CPCM,Solvent=chloroform) Freq NMR EmpiricalDispersion=GD3. The input file was generated and uploaded onto the HPC system for calculations. The 1H and 13C NMR spectra of compound 17 DOI:10042/26345 and compound 18 DOI:10042/26113 were then obtained after a couple of hours. The data obtained from calculations were compared with literature values[7].
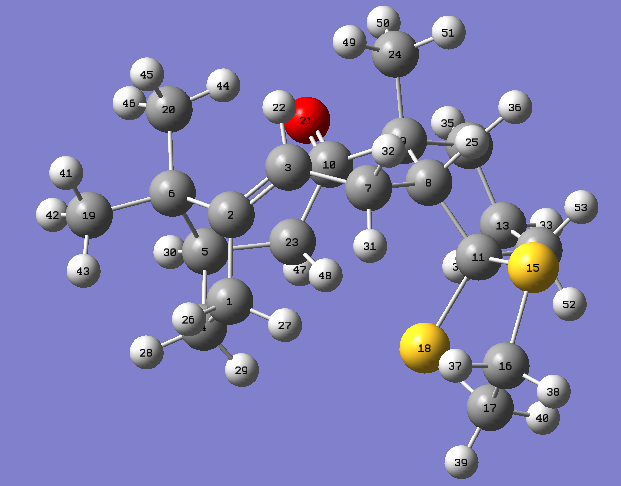
Molecule 17
A gaussview image of molecule 17 after HPC calculations:
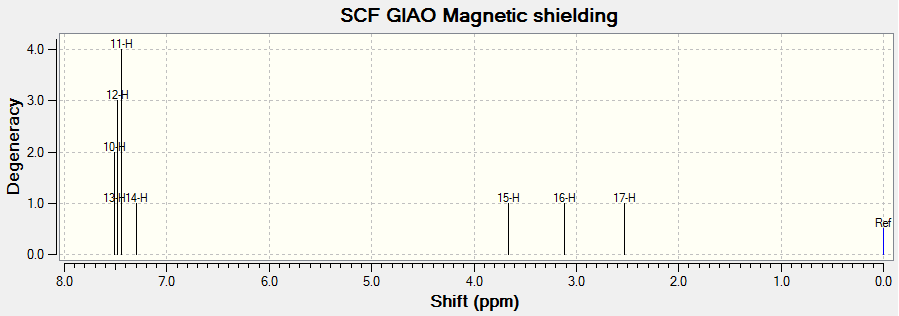
Setting the reference as TMS B3LYP/6-31G(d,p) Chloroform, the following NMR spectra where obtained.
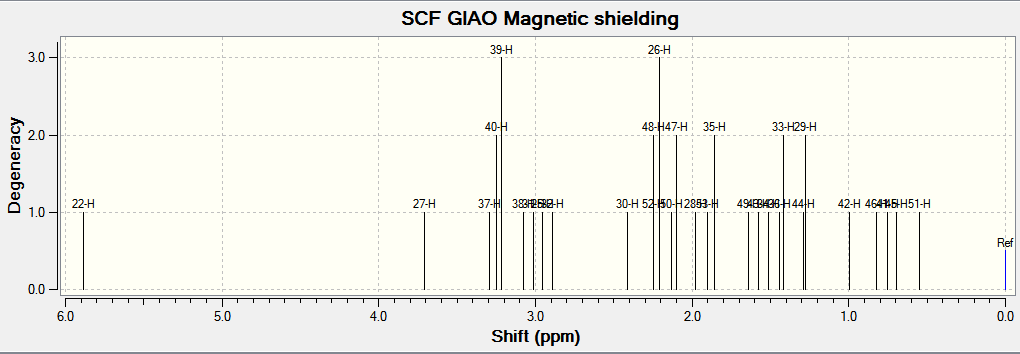
1H NMR
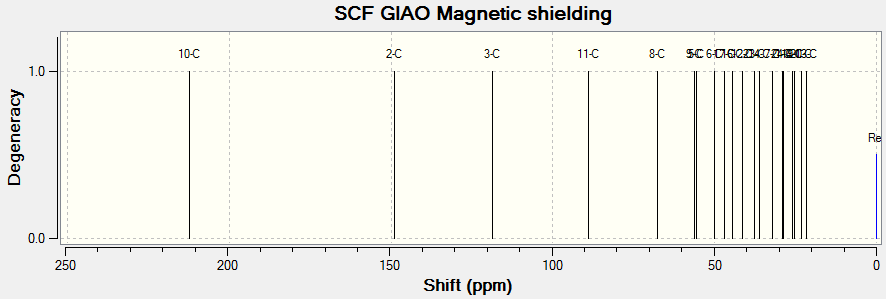
(Reference shielding: 31.7462 ppm, NMR Degeneracy Tolerance: 0.05)
Table of comparison between NMR shifts calculated and literature values
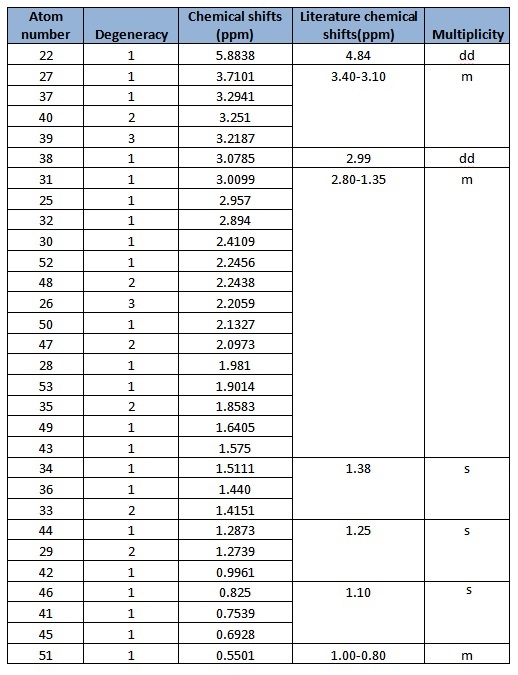
In general, the chemical shifts in the 1H NMR spectra obtained via computational simulations are rather similar to that in literature, except for a few atoms. In particular, atom 22 deviates most from literature. This might be due to the position of H present in the molecule that makes it chemically equivalent but magnetically inequivalent. Also, atoms 49, 50 and 51 are all in the same environment yet atom 51 is given such a low chemical shift compared to the other two atoms. This might indicate an error in the reported chemical shifts. In addition, there are individual chemical shifts reported for each of the atoms, yet in literature, they were reported as a multiplet. This shows that computational simulations could actually give much more accurate results in terms of the chemical shift for each atom.
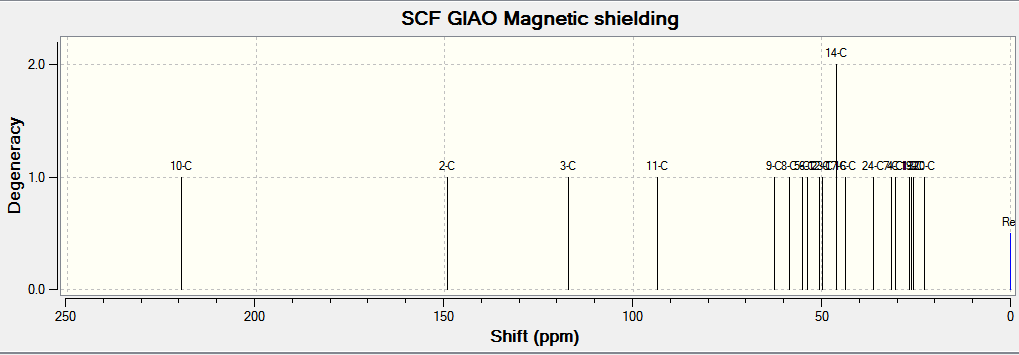
(Reference shielding: 192.17 ppm, NMR Degeneracy Tolerance: 0.05)
Table of comparison between NMR shifts calculated and literature values
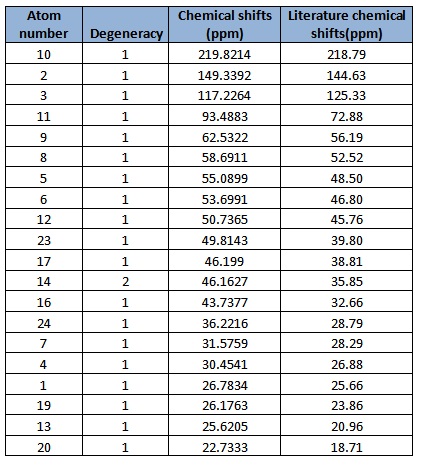
In general, the chemical shifts in the 13C NMR spectra obtained via computational simulations are rather similar to that in literature. However, it is good to note that there is a large deviation in computed results and literature in atom 11. This might be because atom 11 is between two sulfur atoms which are much heavier compared to carbon. In order for a more accurate value of chemical shift, corrections and other calculations need to be made in order to remove the error caused by spin-orbit coupling.
Molecule 18
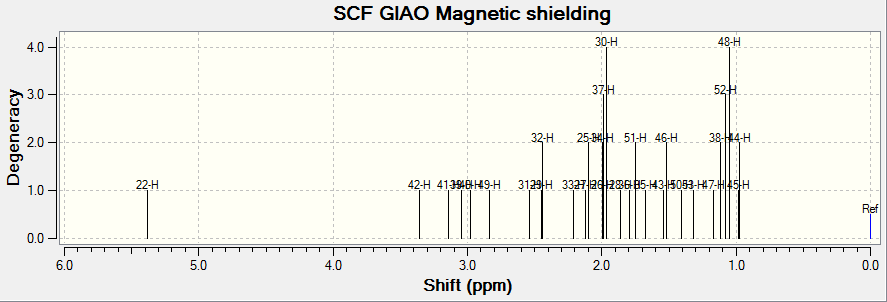
1H NMR
(Reference shielding: 31.7462 ppm, NMR Degeneracy Tolerance: 0.05)
Table of comparison between NMR shifts calculated and literature values
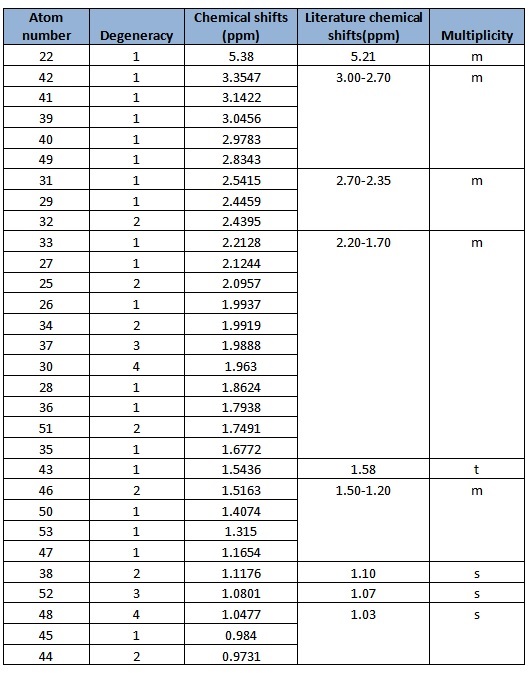
In general, the chemical shifts in the 1H NMR spectra obtained via computational simulations are rather similar to that in literature, except for a few atoms. However, there are some errors reported in literature as well. Atoms 51, 52 and 53 are in the same environment and hence should appear as a singlet, yet for atoms 51 and 53, it is reported as a multiplet. In addition, there are individual chemical shifts reported for each of the atoms, but they were reported as a multiplet in literature. This shows that computational simulations could actually give much more accurate results in terms of the chemical shift for each atom
13C NMR
(Reference shielding: 192.17 ppm, NMR Degeneracy Tolerance: 0.05)
Table of comparison between NMR shifts calculated and literature values

In general, the chemical shifts in the 13C NMR spectra obtained via computational simulations are rather similar to that in literature. Similar to molecule 17, it is good to note that there is a large deviation in computed results and literature in atom 11. This might be because atom 11 is between two sulfur atoms which are much heavier compared to carbon. In order for a more accurate value of chemical shift, corrections and other calculations need to be made in order to remove the error caused by spin-orbit coupling.
Comparison of thermodynamic quantities of molecules 17 and 18
From the .log file obtained, the thermodynamic quantities obtained are shown as below:
Comparing the "Sum of electronic and thermal Free Energies" of the molecules 17 and 18, it is observed that molecule 18 has a slightly lower energy than molecule 17, hence implying that molecule 18 is more stable.
Analysis of the properties of the synthesised alkene epoxides
Objectives for Spectroscopic simulations
The Shi and Jacobsen asymmetric epoxidation catalysts were used to product two different chiral alkene epoxides of unknown absolute configurations in an enantiomeric excess. The configuration of the products were predicted and rationalised by observing the catalyst structure, product NMR spectra, absolute configuration and interactions.
Crystal structure of pre-catalysts
Using Conquest, the pre-catalysts 21 and 23 are searched on the Cambridge crystal database. Pre-catalyst 21 was found to be NELQEA, and pre-catalyst 23 was found to be TOVNIB01.
Pre-catalyst 21
The typical C-O bond length is 1.43Å. However, due to orbital overlap, the bond lengths obtained from the crystal structure above depict some deviations from the typical bond length. Here, the sp3 orbital of carbon overlaps with the π* orbital of oxygen, leading to a decrease in C-O bond order, hence weakening the C-O bond, causing the bond length to be larger than 1.43Å. (At the same time, this effect strengthens the C-C bond attached to the C-O group.) There are also some bond lengths which are observed to be shorter than 1.43Å - one possible reason would be resonance, where the lone pair of electrons on oxygen are available for donation into the adjacent bonds. As a result, the bond would have partial double bond character, increasing the bond strength as compared to just purely a single bond.
Pre-catalyst 23
From the diagram above, it is observed that there is a close approch of the two adjacent t-Bu groups. The shortest distance between a pair of hydrogens which are not bonded to each other was found to be 2.02Å and 2.42Å for the two crystal structures. This is similar to the Van Der Waals radii for maximum attractive interaction of 2.42Å, hence indicating that this attractive interaction helps to stabilise the molecule.
Prediction of NMR of products
Under "Extensions", Gaussian was then selected. The calculation was set to "Geometry optimisation", B3LYP with basis set 6-31G(d,p). The keyword line was set to be: # B3LYP/6-31G(d,p) Opt SCRF=(CPCM,Solvent=chloroform) Freq NMR EmpiricalDispersion=GD3. The input file was generated and uploaded onto the HPC system for calculations. The 1H and 13C NMR spectra of compound trans-stilbene oxide DOI:10042/26093 and styrene oxide DOI:10042/26094 were then obtained after a couple of hours. The data obtained from calculations were compared with literature values [8].
Trans-stilbene epoxide
| Property | Trans-stilbene epoxide | ||
|---|---|---|---|
39.45692 kcal/mol |
Under "Extensions", Gaussian was then selected. The calculation was set to "Geometry optimisation", B3LYP with basis set 6-31G(d,p). The keyword line was set to be: # B3LYP/6-31G(d,p) Opt SCRF=(CPCM,Solvent=chloroform) Freq NMR EmpiricalDispersion=GD3. The input file was generated and uploaded onto the HPC system for calculations. The 1H and 13C NMR spectraDOI:10042/26093 was obtained after a couple of hours.
1H NMR
(Reference shielding: 31.7462 ppm, NMR Degeneracy Tolerance: 0.05)
Table of comparison between NMR shifts calculated and literature values

In general, the chemical shifts in the 1H NMR spectrum obtained via computational simulations are rather similar to that in literature. The multiplet (chemical shifts of around 7.50ppm) are observed for the protons in the benzene ring, while the singlet at around 3.50ppm corresponds to the protons attached to the carbon directly bonded to the epoxide.
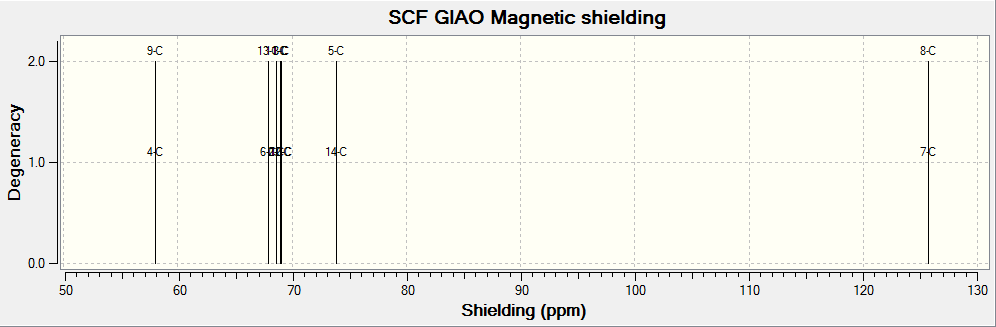
(Reference shielding: 192.17 ppm, NMR Degeneracy Tolerance: 0.05)
Table of comparison between NMR shifts calculated and literature values
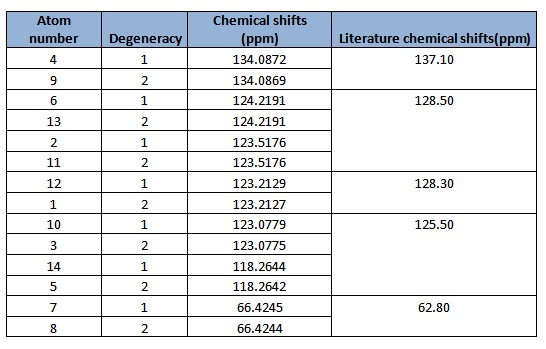
In general, the chemical shifts in the 13C NMR spectrum obtained via computational simulations are rather similar to that in literature. The chemical shifts above 100ppm attribute to those carbons in the benzene ring, whereas the chemical shift of about 66ppm corresponds to the carbon bearing the epoxide.
Styrene epoxide
| Property | Styrene epoxide | ||
|---|---|---|---|
21.61510 kcal/mol |
Under "Extensions", Gaussian was then selected. The calculation was set to "Geometry optimisation", B3LYP with basis set 6-31G(d,p). The keyword line was set to be: # B3LYP/6-31G(d,p) Opt SCRF=(CPCM,Solvent=chloroform) Freq NMR EmpiricalDispersion=GD3. The input file was generated and uploaded onto the HPC system for calculations. The 1H and 13C NMR spectraDOI:10042/26094 was obtained after a couple of hours.
1H NMR
(Reference shielding: 31.7462 ppm, NMR Degeneracy Tolerance: 0.05)
Table of comparison between NMR shifts calculated and literature values
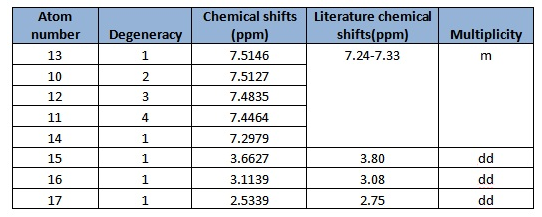
In general, the chemical shifts in the 1H NMR spectra obtained via computational simulations are rather similar to that in literature. The chemical shifts of about 7ppm correspond to those in the benzene ring, while the atom 16 and 17 correspond to that at the terminal end of the epoxide. Atoms 16 and 17 have similar shifts as they are both at the terminal end of the epoxide, compared to atom 15 which has the carbon bonded to the benzene ring. A doublet of doublet is observed as the protons are chemically equivalent but not magnetically equivalent, and this agrees with the literature data found.
(NMR Degeneracy Tolerance: 0.05)
Table of comparison between NMR shifts calculated and literature values

In general, the chemical shifts in the 13C NMR spectrum obtained via computational simulations are rather similar to that in literature. The chemical shifts above 100ppm attribute to those carbons in the benzene ring, whereas the chemical shift of about 54ppm corresponds to the carbon bearing the epoxide.
Assigning the absolute configuration of the product
The two alkenes chosen are trans-stilbene and styrene. The four possible configurations are shown below:

The reported literature for optical rotations
From previous literature (the same ones used as above), it was concluded that the styrene oxide drawn for the NMR analysis is of (R)-configuration, while the trans-stilbene oxide drawn for the NMR analysis is of (R),(R)-configuration.
However, regardless of (R) or (S) configurations, the NMR yielded is the same.
The calculated chiroptical properties of the product
Optical Rotation
Trans-stilbene oxide
For (R),(R)-configuration: The optical rotation obtained (5890.0 A) = 182.37o, ( 3650.0 A) = 687.26o.
Comparing with literature[9] (5890.0 A), the optical rotation value = 205o.
For (S),(S)-configuration: The optical rotation obtained (5890.0 A) = -184.08o, ( 3650.0 A) = -693.97o.
Comparing with literature[10] (5890.0 A), the optical rotation value = -205.2o.
Styrene oxide
For the (R)-configuration: The optical rotation obtained (5890.0 A) = 25.92o, (3650.0 A) = 30.70o. Comparing with literature[11] (5890.0 A), the optical rotation value = 21.5o.
For the (S)-configuration: The optical rotation obtained (5890.0 A) = -25.26o, (3650.0 A) = -28.17o. Comparing with literature[12] (5890.0 A), the optical rotation value = -33.2o.
It is observed that the (R)-configurations give a positive optical rotation value while the (S)-configurations give a negative optical rotation value.
For both oxides, there is a significant amount of deviation from literature values to the values obtained via computational methods. This is possibly due to the fact that the molecules are drawn with different conformations (a slightly tilted epoxide relative to the R groups might have a significant impact on the OR values - especially since these values are dependent on path length, and the path lengths from various literature do vary). However, it might also be possible that the literature values are not very accurate. As epoxides are formed in a racemic mixture in the experiment, they have to be separated before characterisation. In this process, there might be residues of the other conformation of epoxides left in the supposedly enantiomerically-pure container, resulting in some differences between literature and calculated OR values.
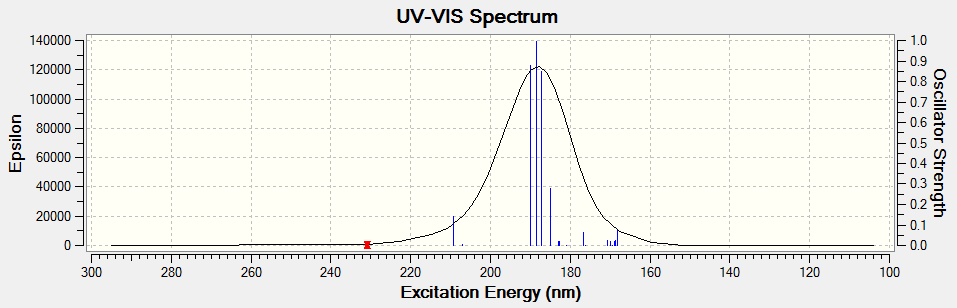
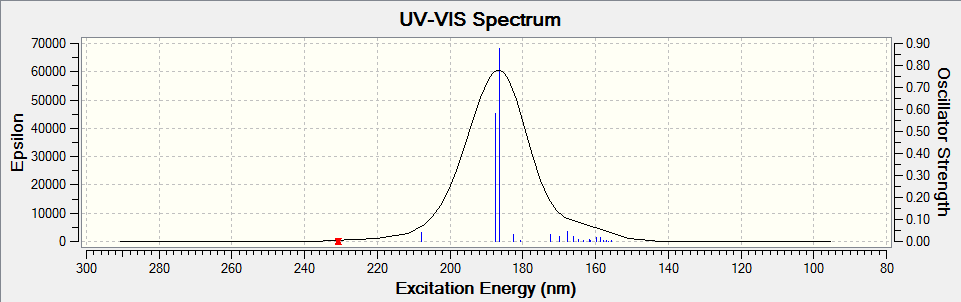
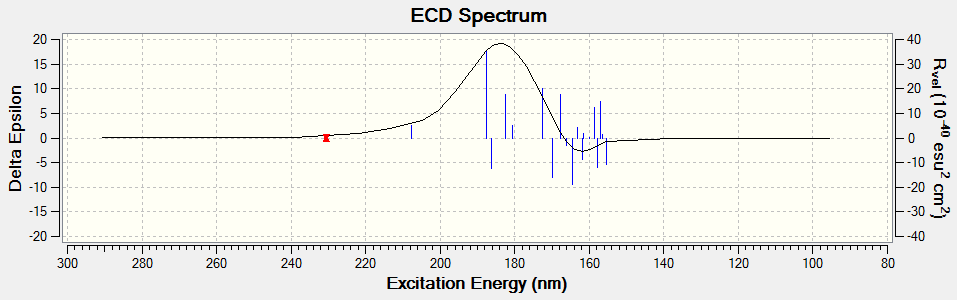
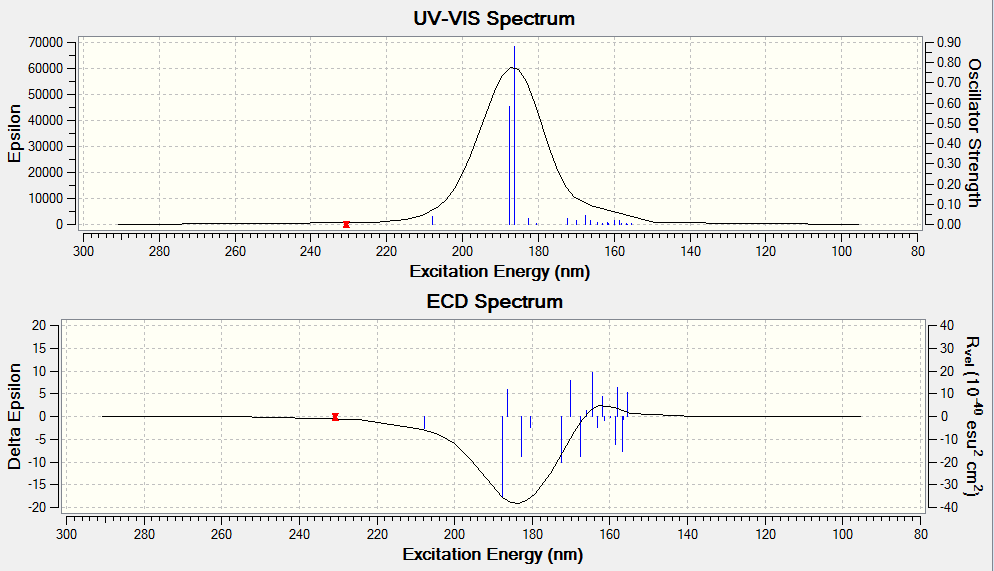
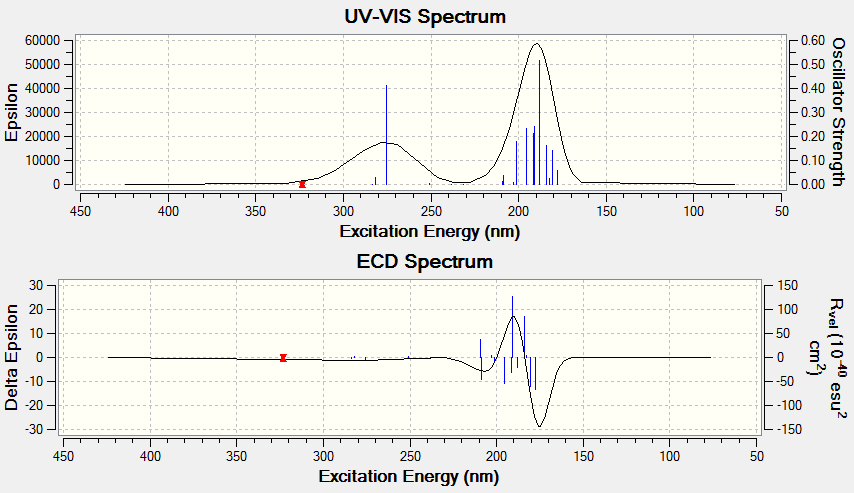
Electronic circular dichroism (ECD)
The ECD is the UV/Vis spectrum recorded with polarised light. Although there are no chromophores in the epoxides, the spectra are still recorded and calculated. It is observed that for the alkene oxides with opposite configurations, ie (R) versus (S) configuration, the ECD spectrum of one configuration is in fact a reflection about the x axis of the other configuration. This would be useful in determining the (R) and (S) configuration of a given compound.
Trans-stilbene oxide
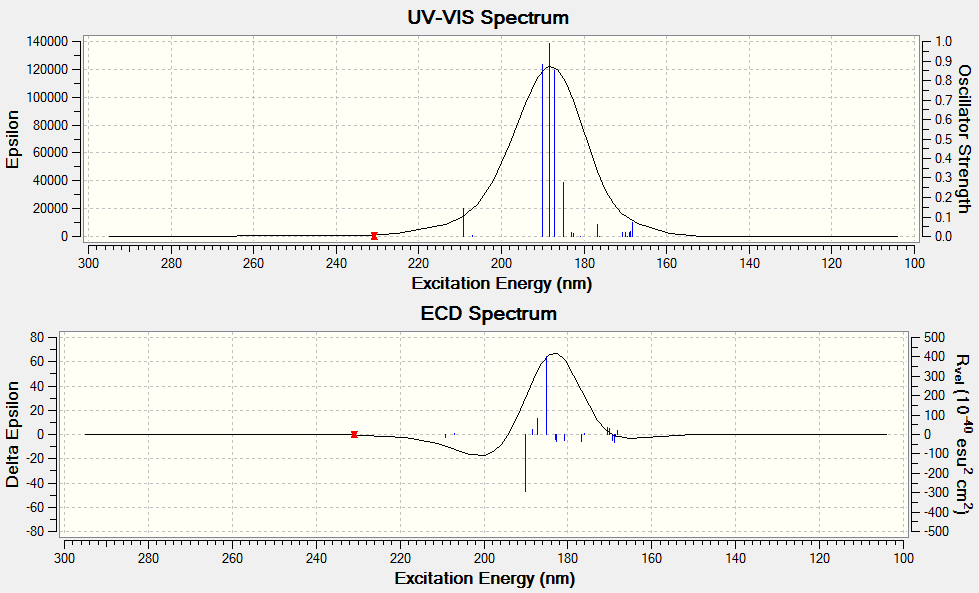
Styrene oxide
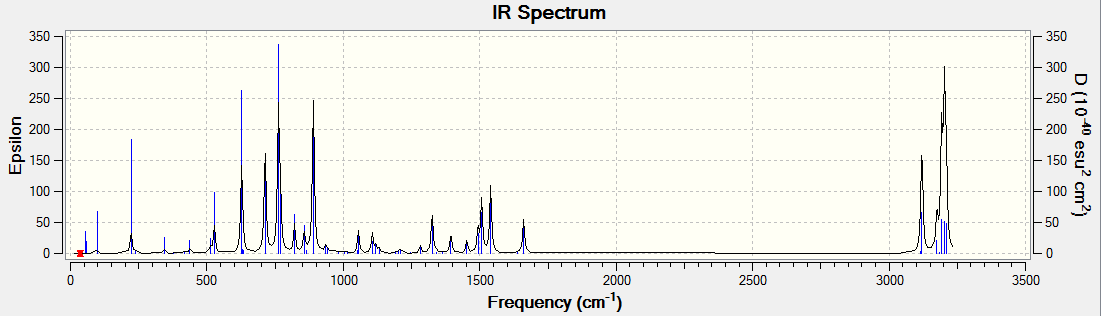
Vibrational circular dichroism (VCD)
It is observed that for the alkene oxides with opposite configurations, ie (R) versus (S) configuration, the VCD spectrum of one configuration is in fact a reflection about the x axis of the other configuration. This would be useful in determining the (R) and (S) configuration of a given compound.
Trans-stilbene oxide
Styrene oxide
Using the calculated properties of transition state for the reaction
The relative computed free energy of the transition state can be used to check for enantiomeric assignments via comparison of computed and calculated optical rotations. Since ∆G =-RTlnK, where R is the gas constant (8.3145JK-1mol-1), T is the temperature (298.15K), and K = (1-X)/X, where X is the mole fraction of one of the isomers. The enantiomeric excess is the difference between the mole fractions of the two isomers.
Note that in the calculations below, the following conversion is used: 1 hartree = 2 625.5 J/mol.
Transition states for Shi epoxidation of trans-stilbene
The sum of electronic and thermal free energies of all the data given were used in the calculations.
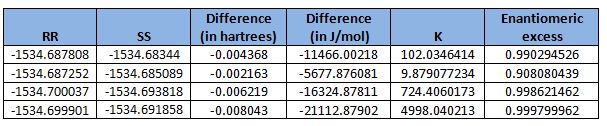
From the table shown, the (R),(R)-configuration is favoured in all transition states. This is in agreement with literature[13], where the enantiomeric excess was reported to be 97% (ie (R),(R)-configuration is favoured).
Transition states for Shi epoxidation of styrene

From the table shown, for the first and forth transition state, the (S)-configuration is favoured, but for the second and third transition states, the (R)-configuration is favoured. This is in agreement with literature[14], where the enantiomeric excess was reported to be 99% (ie (S)-configuration is favoured).
Transition states for Jacobsen epoxidation of cis-β-methyl styrene
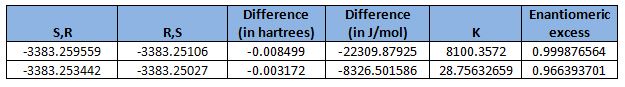
From the table shown, it is clearly observed that the (S),(R)-configuration is favoured. This is in agreement with literature[15], where the enantiomeric excess was reported to be 92%. Although the calculated value is higher than the value reported in literature, the fact that the (S),(R)-configuration is favoured still holds true.
Transition states for Jacobsen epoxidation of trans-β-methyl styrene

From the table shown, it is clearly observed that the (S),(S)-configuration is favoured. This is in agreement with literature[16], where the enantiomeric excess was reported to be 92%. Although the calculated value is higher than the value reported in literature, the fact that the (S),(S)-configuration is favoured still holds true.
Investigating the non-covalent interactions in the active-site of the reaction transition state
Non-covalent interactions (NCI) include hydrogen bonds, electrostatic attractions and dispersion-like close approaches of pairs of atoms. NCI intereactions can be defined by properties of the electron density of the molecule of interest for normal covalent bonds.
In the diagram shown, blue represents very attractive interactions, green represents a mildly attractive interaction, yellow represents mildy repulsive and red represents strong repulsive interactions.
Here, the R series of styrene oxide transition state is chosen for this analysis.
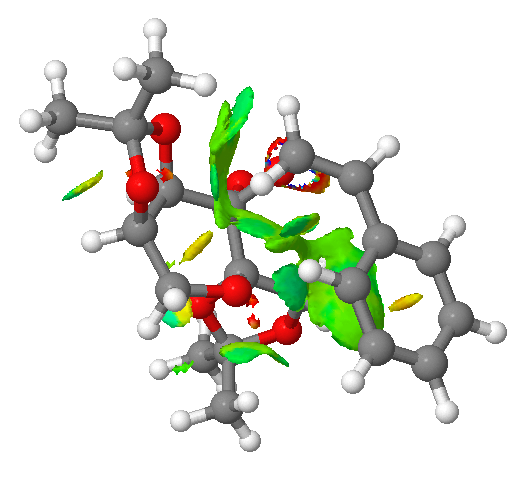
The center of the Shi catalyst is placed next to the alkene of the styrene. As shown in the diagram, there is a large patch of green electron density, which is indicative of mildly attractive interactions between those two molecules. The oxygen atom in O-C-O bond (not involved in the epoxidation reaction) has a favourable interaction with the hydrogen (at the bottom of the diagram) - intramolecular hydrogen bonding. In addition, the hydrogen from the methyl group in the Shi catalyst (top of the diagram) shows a favourable interaction (green) with the hydrogen atom of the alkene group in styrene. Combined, all these favourable and attractive interactions lead to the overall stabilisation of the molecule system.
Investigating the Electronic topology (QTAIM) in the active-site of the reaction transition state
This technique focuses on electron density and its curvature in the covalent regions of the molecules and the weaker interactions which were previously identified via the NCI analysis. A bond critical point (BCP) is defined by a region where the first derivative of the electron density with respect to each of the three coordinates of that point is zero which has a curvature[17] Here, the R series of styrene is used for analysis.
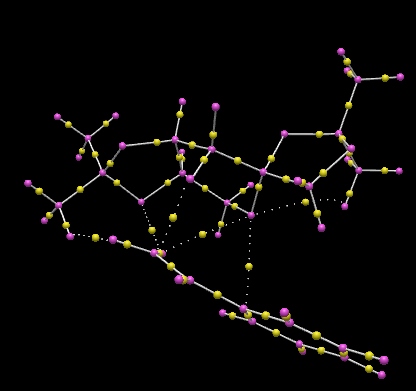
From the QTAIM diagram above, it can be observed that the oxygen atom involved in the epoxidation reaction has weak non-covalent interactions with the carbon atom of the alkene, and this is suggestive of how the epoxidation reaction occurs. There is also some non-covalent interactions between the oxygen atoms in O-C-O (those not involved in the epoxidation reaction) and the Shi catalyst, as seen by the dotted lines, but not all the oxygens have such interactions. Apart from these interactions, the stabilisation of the entire molecule is due to hydrogen bonding (oxygens (of O-C-O) are stabilised by interacting with a hydrogen atom) and also the weak Van der Waals forces of attractions between atoms as shown by the BCPs.
Discussion
There are some limitations using QTAIM analysis and from previous literature[18], it can be seen that QTAIM is not fully reliable. For instance, there are examples where BCP is not observed for an intuitive bond (common for intramolecular hydrogen bonds), or BCP is observed when there is no apparent bonding (common for repulsive H···H steric interactions especially for biphenyl derivatives for example). Hence, the absence of BCP does not definitely mean the abscence of a chemical bond, vice versa.
However, in this experiment, the QTAIM data obtained is still rather reliable in suggesting an explanation of how the epoxidation reaction proceeds.
Suggesting new candidates for investigation
One possible candidate for investigation is 4-nitrostilbene, and the reaction is shown below. The optical rotation value[19] is 3.5° at 589nm. This alkene is suggested as it is a derivative of the trans-stilbene used previous, however, there is an addition of a electron-withdrawing group at the para position. Hence, it would be interesting to see how an electron withdrawing group can affect the optical properties, spectra, energies, site and rate of approach of the Shi and Jacobsen catalyst to the alkene. Apart from these interesting properties to be searched on, this alkene is also available on Sigma-Aldrich, which is why it was chosen.
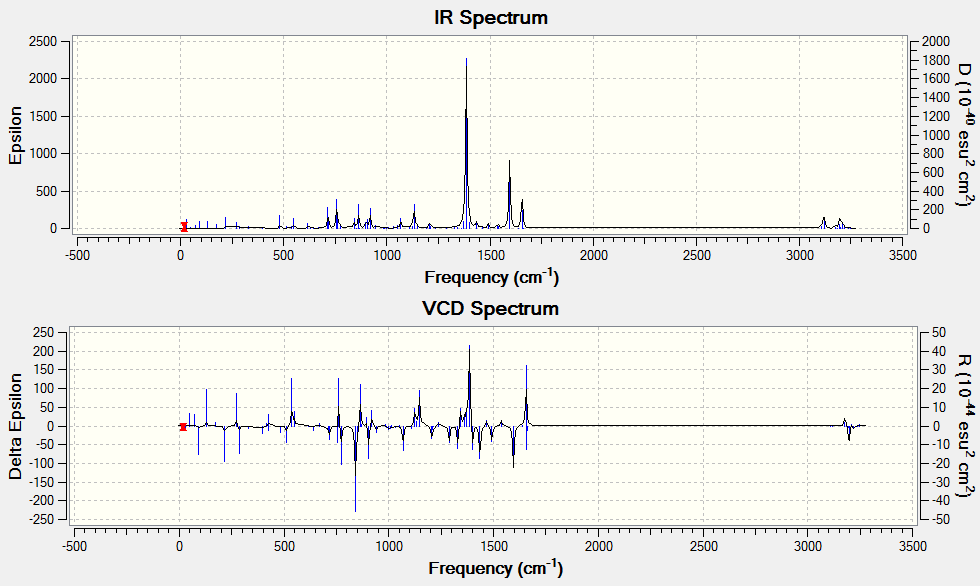
Additional Calculations
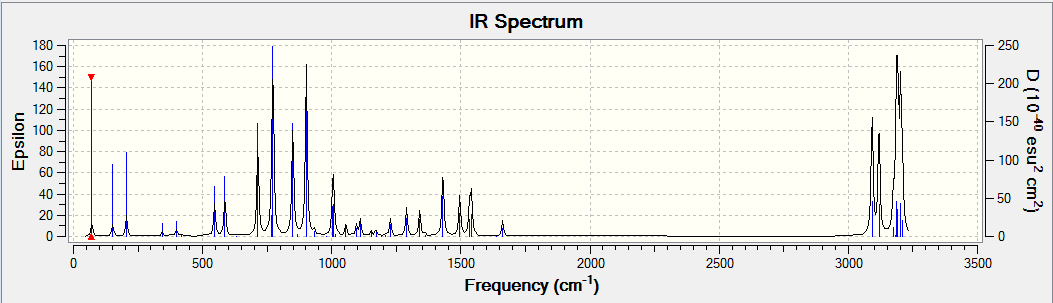
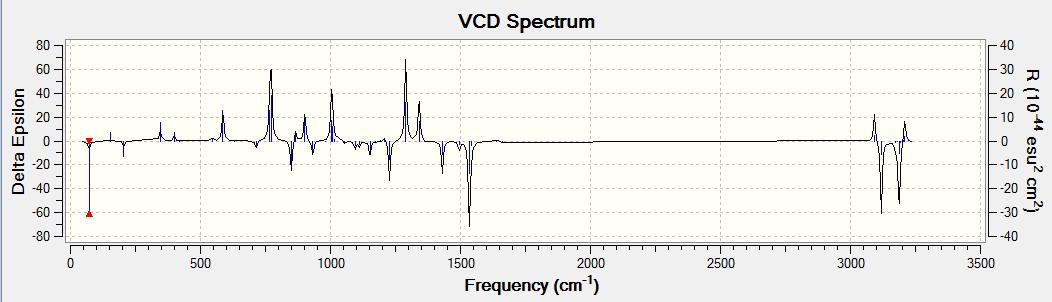
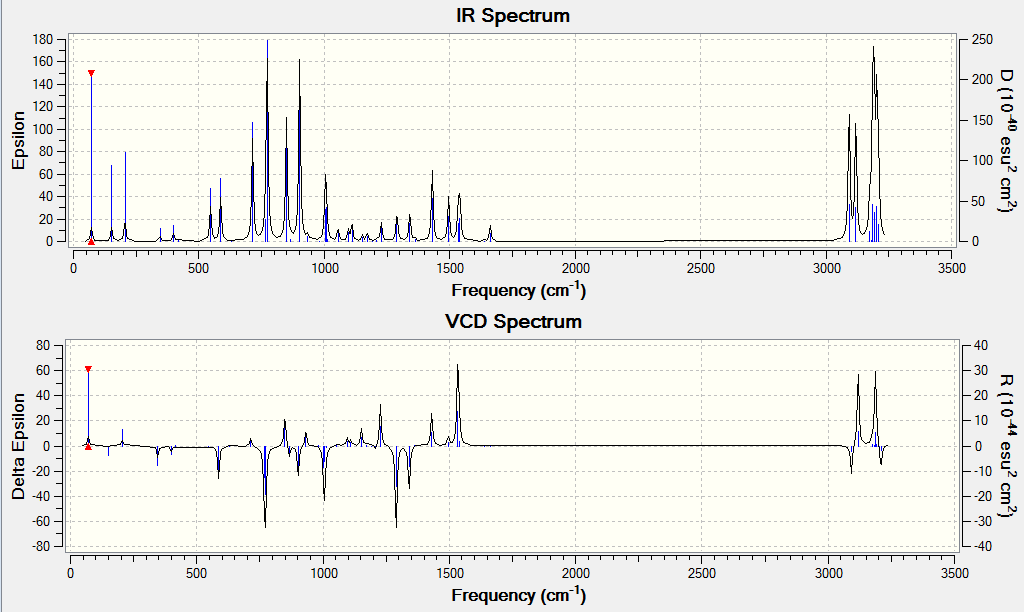
The optical rotation, ECD and VCD spectra was obtained by generating a code from the Gaussian function in Avogadro before submitting to HPC. The results obtained are shown below. The alkene used for analysis has (R),(S)-configuration.
The optical rotation obtained is: -67.52° (5890.0 A), -498.49° (3650.0 A)
DOI:10042/26452
DOI:10042/26440
VCD
DOI:10042/26451
Conclusion
In this experiment, computational simulations were used to calculate energies, transition states and spectra of the epoxidation reactions using Shi and Jacobsen catalysts. The spectra data were rather close to those of literature values, hence indicating the high accuracy computational methods, which is very useful for future prediction of spectra or energies of molecules. In fact, using computational methods produce more accurate data than experimental and literature values. Although there are some limitations (as mentioned in the introduction), computational methods are still considered pretty accurate. As an additional confirmation of the accuracy of the results obtained via computational simulations, more methods and higher level of theories can be used, and compared between sets of data to check for consistency and reproducibility of results.
References
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ Bringmann G, Mortimer AJP, Keller PA, Gresser MJ, Garner J, Breuning M, Atroposelective Synthesis of Axially Chiral Biaryl Compounds, 2005, 44; DOI:10.1002/anie.200462661
- ↑ See J. G. Vinter and H. M. R. Hoffman, J. Am. Chem. Soc., 1974, 96, 5466–5478 (DOI:10.1021/ja00824a025 )
- ↑ W. F. Maier, P. Von Rague Schleyer, J. Am. Chem. Soc., 1981, 103, 1891. DOI:10.1021/ja00398a003
- ↑ Liebman, J. F.; Greenberg, ACS Publications, 31, 76, 1976. DOI:10.1021/cr60301a002
- ↑ Pelayo Camps, Francesc Pérez, Santiago Vázquez, Elsevier, 53, 28, 1997. DOI:10.1016/S0040-4020(97)00595-4
- ↑ Spectroscopic data: L. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, R. D. Rogers, J. Am. Chem. Soc.,, 1990, 112, 277-283. DOI:10.1021/ja00157a043
- ↑ Charlotte Wiles, Marcus J. Hammond and Paul WattsJ. Org. Chem.,, 2009 DOI:10.3762/bjoc.5.27
- ↑ Solladie-Cavallo, Arlette; Diep-Vohuule, Anh; Sunjic, Vitomir; Vinkovic, Vladimir , Tetrahedron Letters, 1996, 7; 6; pp1783 - 1788 DOI:10.1016/0957-4166(96)00213-3
- ↑ Niwa, Takashi; Nakada, Mashisa, J. Am. Chem, 2012, 134; 6; pp13538-13541 DOI:10.1021/ja304219s
- ↑ Sarma, Kuladip; Goswami, Amrit; Goswami, Bhabesh C., Tetrahedron Letters, 2009, 20; 11; pp1295 - 1300 DOI:10.1016/j.tetasy.2009.05.001
- ↑ E. J. Corey , Saizo Shibata , Raman K. Bakshi, J. Org. Chem, 1988, 58; 12; pp2861–2863 DOI:10.1021/jo00247a044
- ↑ Wong, O. Andrea; Wang, Bin; Zhao, Mei-Xin; Shi, Yian , J. Org. Chem, 2009, 74; 16; pp6335-6338 DOI:10.1016/j.tetasy.2009.02.035
- ↑ Schrittwieser, Joerg H.; Kroutil, Wolfgang; Lavandera, Ivan; Seisser, Birgit; Mautner, Barbara; Lutje Spelberg, Jeffrey H., Tetrahedron Letters, 2009, 20; 4; pp483-488 DOI:10.1016/j.tetasy.2009.02.035
- ↑ Eric N. Jacobsen , Wei Zhang , Alexander R. Muci , James R. Ecker , Li Deng, J. Am. Chem. Soc., 1991, 113; 18; pp7063–7064 DOI:10.1021/ja00018a068
- ↑ Adrian M. Daly , Marie F. Renehan , and Declan G. Gilheany, J. Am. Chem. Soc., 2001, 3; 5; pp663–666 DOI:10.1021/ol0069406
- ↑ Professor Henry Rzepa, Imperial College London Laboratory script, 2013.
- ↑ Joseph R. Lane, Julia Contreras-García, Jean-Philip Piquemal, Benjamin J. Miller, and Henrik G. Kjaergaard. , J. Chem. Theory Comput., 2013, 9; 3263-3266 DOI:10.1021/ct400420r
- ↑ Dansette,P.M. et al., Tetrahedron, 1976, 32; pp2071 - 2074 DOI:10.1016/0040-4020(76)85110-1