Rep:Mod:3GPPHYS
Module 3
The Cope Rearrangement
The Cope reaction like all pericyclic reactions is known from literature to go through a concerted transition state, the reaction has no intermediates[1]. The cope reaction involves a [3,3] concerted sigmatropic shift of the 1,5 hexadiene molecule through a benzene like transition state. There is a (4n+2) electron count and the reaction proceeds suprafacially under thermal conditions by either a chair or boat structured transition state with the chair conformation being the lower in energy see transition state analysis below

Optimizing the Reactants and Products
[D space 1,5-hexadiene "anti" optimization]
Item Value Threshold Converged?
Maximum Force 0.000056 0.000450 YES
RMS Force 0.000010 0.000300 YES
Maximum Displacement 0.001317 0.001800 YES
RMS Displacement 0.000459 0.001200 YES
Predicted change in Energy=-2.090808D-08
Optimization completed.
-- Stationary point found.
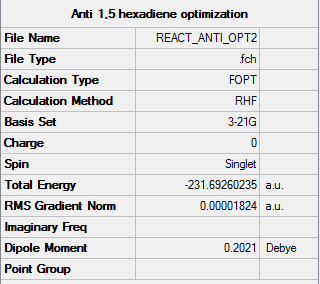
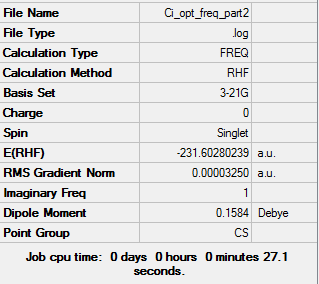
A molecule of 1,5-hexadiene with one anti linkage was optimized using the Hartree Fock method and the 3-21G basis set. The Item table above shows that a minimum for the calculation was successfully reached.
As can be seen from the summary table above the data obtained from the optimization of the 1,5 hexadiene molecule matches that expected for an anti 1 structure as shown by the table in the tutorial wiki [2]. The point group of the calculated molecule is C2 and the computed energy is -231.69260(235) (relative energy). These match the literature for a Anti-1 conformer molecule [2]
Optimization of 1,5-hexadiene with a "gauche" linkage
[D space 1,5-hexadiene "gauche" optimization]
Item Value Threshold Converged?
Maximum Force 0.000019 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000737 0.001800 YES
RMS Displacement 0.000163 0.001200 YES
Predicted change in Energy=-2.588575D-08
Optimization completed.
-- Stationary point found.
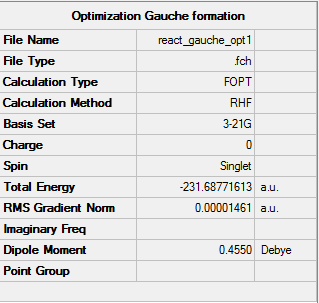
The Item table shows that the calculations have converged and the calculation has successfully reached a minimum.
As can be seen from the summary table above the molecule has C2 symmetry and an energy of -231.68771(613) both of these are consistent with the data quoted in the instruction wiki for a gauche 1 conformation [2]
Considering the 1,5 hexadiene in the same way as you would consider the saturated structure would expect the anti structure to be lower in energy relative to the gauche structure. As there will be less eclipsed groups and therefore less pauli repulsion energy. There are also favorable orbital overlaps in the form of σC-H/σ*C-H-orbital interactions and σC-C/σ*C-C-orbital interactions. As expected for the anti 1 and gauche 1 conformation the anti formation is lower in energy. [3] However it can be seen that the lowest energy structure for the 1,5 hexadiene molecule was the gauche 3 conformation which when just considering σ interactions is unexpected. The stability of the gauche 3 structure is probably due to favourable pi orbital and vinyl proton interactions made possible by the 1, 5 c=c bonds along with the favourable van der waal H-H attractions seen in the structure with more eclipsed elements outweighing the effect of the increased pauli repulsion effect. [4] The gauche 3 conformation is the lowest energy structure as it shows a minimum between the gauche 2 structure and the gauche 4 structure, the energy does not get lower with any more increase in eclipsed components. There are 3 C-C bonds in the 1,5 hexadiene structure with free rotation, this allows 27 different theoretical conformations however due to symmetry there are actually only 10 different conformations, all of these conformations have been accounted for on the table shown in the instructions wiki showing that there cannot be a lower energy conformation of 1,5 hexadiene then the gauche 3 conformation.
1,5-hexadiene Ci anti 2 optimization HF/3-21G level
[D space 1,5-hexadiene Ci anti 2 optimization HF/3-21G level]
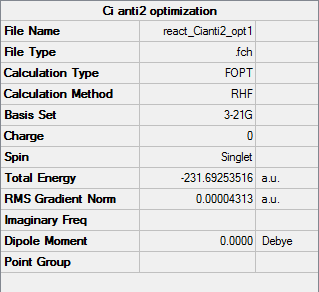
The anti 2 conformation of hexa1,5diene was optimized initially using the the 3-21G basis set and the HF method.
Item Value Threshold Converged?
Maximum Force 0.000110 0.000450 YES
RMS Force 0.000022 0.000300 YES
Maximum Displacement 0.000688 0.001800 YES
RMS Displacement 0.000235 0.001200 YES
Predicted change in Energy=-1.327343D-07
Optimization completed.
-- Stationary point found.
The calculation converged showing a minimum was reached for the calculation.
The final energy calculated for the anti 2 conformation was -231.69253(516) au and the symmetry was found to be Ci this is consistent with the the values for the anti 2 conformation shown in the table on the information wiki [2], which shows an energy of -231.69254 au my calculation has a very similar value to the energy value provided suggesting high accuracy of my calculation even using the low level 3-21G basis set.
1,5-hexadiene Ci anti 2 optimization and frequency B3LYP/6-31G* level
[D space 1,5-hexadiene Ci anti 2 optimization B3LYP/6-31G* level]
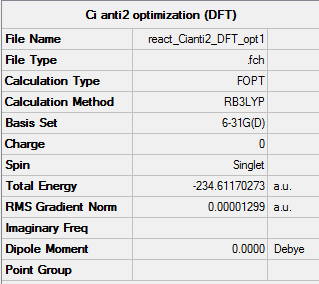
The anti 2 conformer was then re optimized at the higher level basis set of 6-31G* using the B3LYP method, a frequency calculation was also performed.
Item Value Threshold Converged?
Maximum Force 0.000016 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000215 0.001800 YES
RMS Displacement 0.000079 0.001200 YES
Predicted change in Energy=-1.572608D-08
Optimization completed.
-- Stationary point found.
The item table shows the optimization converged and minimum was successfully found.
| Comparison of anti 2 conformation optimization | 3-21G basis set | 6-31G* basis set |
|---|---|---|
| Energy au | -231.69253(516) | -234.61170(273) |
| Geometry |  |

|
The energy calculated using the B3LYP/6-31G* method and basis set is significantly different from that calculated using the HF/3-21G calculation being much lower, because the energies were calculated using different basis sets they are not directly comparable. There is a difference in energy of 2.91916 au which is equal to 1831 kcal/mol. The geometries of both optimizations are very similar and there are only negligible differences in the bond lengths and bond angles between the 2 structures. The HF/3-21G calculation has a C=C bond length of 1.32 A a C-C bond length of 1.51 A, a C-H bond length of 1.07 A and a C-C-H sp2 hybridised bond angle of 121.8o. The B3LYP/6-31G* has a C=C bond length of 1.33 A, a C-C bond length of 1.50 A, a C-H bond length of 1.09 A and a C-C-H sp2 hybridised bond angle of 121.7o. There is only 1 A difference between all of the bond lengths and 1 degree between the bond angles.
Anti 2 1,5-hexadiene frequency calculations
[D space 1,5-hexadiene Ci anti 2 frequency B3LYP/6-31G* level]
Low frequencies --- -18.8144 -11.7181 -0.0007 -0.0006 -0.0005 1.7515 Low frequencies --- 72.7146 80.1401 120.0114
The top low frequencies line shows minimal center of mass motion showing the calculation has a good degree of accuracy. The vibrational frequencies of the molecule are all positive showing the molecule is optimized to minimum energy.
Below if a generated IR spectrum for the anti 2 conformation of 1,5-hexadiene.
1,5 hexadiene contains 16 atoms and has 3n-6 vibrational modes therefore there are 42 vibrational modes. Several have been visualised below.
Anti 2 conformer of 1,5-hexadiene Thermochemical data
The below data was extracted from the data read out for the log file of the anti 2 frequency calculation.
Sum of electronic and zero-point Energies= -234.469212 au
Sum of electronic and thermal Energies= -234.461856 au
Sum of electronic and thermal Enthalpies= -234.460912 au
Sum of electronic and thermal Free Energies= -234.500822 au
The first energies value represents the potential energy at 0 Kelvin including the zero point vibrational energy Σ(Eelec + ZPE). The second energy value represents the energy at 298.15 K and 1 atm which is contributed to by vibrational, rotational and translational modes Σ(E + Evib + Erot + Etrans). The third energy value is the same as the second but containing an extra correctional term for RT (H = E + RT) which is particularly important when looking at dissociation reactions. The forth energy value includes the entropic contribution to the free energy (G = H - TS) These calculated values can then be compared to actual experimental data.
Optimizing the "Chair" and "Boat" Transition Structures
In the Cope reaction there are 2 possible transition structures, the lower energy cyclohexane like Chair structure and the higher energy Boat structure. In this section both these transition structures were optimized.
Allyl fragment optimization
An allyl fragment to be used to simulate the 3C sections in the Cope reaction were initially optimized using the HF/3-21G method and basis set. [D space allyl fragment optimization ]
Item Value Threshold Converged? Maximum Force 0.000048 0.000450 YES RMS Force 0.000018 0.000300 YES Maximum Displacement 0.000149 0.001800 YES RMS Displacement 0.000070 0.001200 YES Predicted change in Energy=-1.277266D-08 Optimization completed. -- Stationary point found.
The calculation converged showing that a minimum was successfully found.
Chair transition state Optimization to a TS (Berny)(guess)
2 copies of the allyl fragment where reorientated and translated in a new window to create a guess structure for the chair transition state, the terminal ends of the allyl fragments were placed approximately 2.2 A apart. The molecule was optimized and to a transition state using TS (Berny) as apposed to a minimum and a frequency calculation was also done the Hartree Fock method and basis set 3-21G where again used.
[D space Chair transition state optimization and frequency (guess)]
Item Value Threshold Converged?
Maximum Force 0.000013 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000558 0.001800 YES
RMS Displacement 0.000068 0.001200 YES
Predicted change in Energy=-1.506444D-08
Optimization completed.
-- Stationary point found.
Low frequencies --- -817.9659 -0.9832 -0.0007 -0.0003 0.0001 1.9733 Low frequencies --- 2.5887 209.5368 395.9493 ****** 1 imaginary frequencies (negative Signs) ******
The Item table shows that the calculation converged confirming a minimum was reached, the low frequency values are all positive confirming that a minimum and not just a turning point was reached. There is one imaginary -ve frequency, this corresponds to where in reality the bond is formed and broken. This imaginary vibration is visualized below.
The vibration corresponds to the reaction coordinates of the cope reaction as suggested in theory as expected
Chair transition state Optimization to a TS (Berny)(guess) freeze method
The optimized transition state was found again but using the freeze co ordinate method. The molecule was optimized to a minimum with the atoms where bonding/breaking takes place frozen, the molecule was then optimized to a transition state and a frequency calculation run.
https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/21189 freeze part 1 https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/21269 freeze part 2
Item Value Threshold Converged? Maximum Force 0.000063 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.001470 0.001800 YES RMS Displacement 0.000295 0.001200 YES Predicted change in Energy=-9.596801D-07 Optimization completed.
-- Stationary point found.
The item table shows that a minimum was found in part 1 of the optimization.
Low frequencies --- -818.4372 -8.6744 -5.6707 0.0000 0.0003 0.0007 Low frequencies --- 4.0534 209.5156 396.5659 ****** 1 imaginary frequencies (negative Signs) ******
One imaginary frequency corresponding to the bond breaking/forming reaction coordinates was found with a value of -818.4 cm-1
Imaginary frequency of -818.4372 cm-1
The bond lengths of the reaction co ordinates and the imaginary frequencies are similar for both methods of optimization varying by approximately 1 Armstrong and the imaginary frequencies vary by about 1 nm. The optimized structures also look reasonably similar suggesting that either method of TS optimization is accurate enough to be used. This also suggests that the resulting optimized structure is close to the idea structure.
Boat Transition state optimization using QST2 method
The boat transition structure was optimized using the QST2 method and the optimized Ci anti 2 1,5 hexadiene structure, in this method the reactant and product are specified and labelled the calculation then finds the transition state between them. The TS (Berny) command was used and the transition state was optimized using the HF/3-21G method and basis set.
[[https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/21275 Boat TS optimization
Item Value Threshold Converged? Maximum Force 0.000089 0.000450 YES RMS Force 0.000046 0.000300 YES Maximum Displacement 0.001360 0.001800 YES RMS Displacement 0.000402 0.001200 YES Predicted change in Energy=-1.208600D-06 Optimization completed. -- Stationary point found.
This calculation failed as the reactant and product molecules geometry boat transition structure. Therefore the structures were edited so that the boat could be found by modifying the C2-C3-C4-C5 dihedral angles to 100o and setting C2-C3-C4 and C3-C4-C5 angles to 0o. The optimization was rerun.
[[https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/21278 boat ts frequency
Item Value Threshold Converged?
Maximum Force 0.000105 0.000450 YES
RMS Force 0.000023 0.000300 YES
Maximum Displacement 0.001256 0.001800 YES
RMS Displacement 0.000278 0.001200 YES
Predicted change in Energy=-9.913882D-08
Optimization completed.
-- Stationary point found.
The Item table confirms a minimum was found.
The imaginary vibration is visualized above with a frequency of -839.9 cm-1. This is the same as the vibration seen for the chair transition state and corresponds to the 3,3 sigmatropic shift of the Cope reaction.
IRC calculations
Chair transition structure
It is hard to predict what conformers of 1,5 hexadiene the boat and chair transition structures lead to to solve this problem a calculation with the IRC (Intrinsic Reaction Coordinate) method. The minimum energy path from the transition structure is followed by the calculation down to local minima.
The first optimization for the chair transition state was used, the calculation was run just in the forward direction with the force constant being calculated once with the number of points along the IRC path being specified as 50 and the HF/3-21G method and basis set were used. The calculation did not run to completion, on opening of the file it was found that only 28 out of the 50 possible steps had run. However the the graough of the RMS gradient showed that the Energy was near minization at point already.
The molecule from the 28ths step was then minimised normally using the HF/3-21G method and basis set to ensure a minima, seeing as the calculation is close to a minima already this should give the correct minimum.
Item Value Threshold Converged?
Maximum Force 0.000003 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000204 0.001800 YES RMS Displacement 0.000065 0.001200 YES Predicted change in Energy=-4.373146D-10 Optimization completed. -- Stationary point found.
The item table shows that a minimum of the transition state was successfully found.
Boat transition structure
The QST2 optimized transition state was used for the boat IRC calculation. The calculation was run just in the forward direction with the force constant being calculated once with the number of points along the IRC path being specified as 50 and the HF/3-21G method and basis set were used. Once again the calculation did not complete stopping after 32 steps as apposed to the specified 50 in the calculation. Once again the RMS gradient plot shows that the gradient has almost reached 0 and therefore the calculation was near to a minimum before it was terminated. The molecule from the last step was reoptimized at the HF/3-21G level, this is likely to reach the correct minimum as the calculation is already very near the mimimum value.
Item Value Threshold Converged?
Maximum Force 0.000102 0.000450 YES
RMS Force 0.000016 0.000300 YES
Maximum Displacement 0.000410 0.001800 YES
RMS Displacement 0.000098 0.001200 YES
Predicted change in Energy=-6.475153D-08
Optimization completed.
-- Stationary point found.
The item table shows that a minimum of the transition state was successfully found.
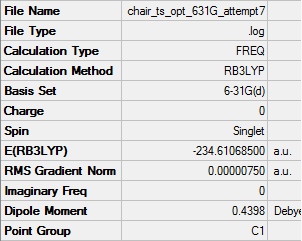
Reoptimisation of chair and boat conformers with 6-31G basis set
Below is the chair conformer reoptimized from the HF/3-21G level to the DFT/6-31G* level, a frequency calculation was also carried out and the data from this calculation was used to calculate the activation energy of the transition state.
test molecule |
https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/21303
Item Value Threshold Converged?
Maximum Force 0.000017 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.001097 0.001800 YES
RMS Displacement 0.000317 0.001200 YES
Predicted change in Energy=-8.462930D-09
Optimization completed.
-- Stationary point found.
Low frequencies --- -18.0329 -14.0948 -10.7685 -0.0003 0.0003 0.0008 Low frequencies --- 63.6803 97.5164 103.7257
Thermochemistry
Sum of electronic and zero-point Energies= -234.468283
Sum of electronic and thermal Energies= -234.460967
Sum of electronic and thermal Enthalpies= -234.460023
Sum of electronic and thermal Free Energies= -234.500013
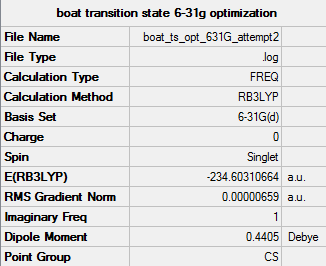
Below is the boat conformer reoptimized from the HF/3-21G level to the DFT/6-31G* level, a frequency calculation was also carried out and the data from this calculation was used to calculate the activation energy of the transition state.
test molecule |
https://spectradspace.lib.imperial.ac.uk:8443/dspace/handle/10042/21306
Item Value Threshold Converged?
Maximum Force 0.000013 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000273 0.001800 YES
RMS Displacement 0.000076 0.001200 YES
Predicted change in Energy=-6.304841D-09
Optimization completed.
-- Stationary point found.
Low frequencies --- -156.9550 -19.9944 -0.0007 -0.0003 0.0005 3.4751 Low frequencies --- 8.3529 75.5006 118.2804
Thermochemistry
Sum of electronic and zero-point Energies= -234.460596
Sum of electronic and thermal Energies= -234.454068
Sum of electronic and thermal Enthalpies= -234.453124
Sum of electronic and thermal Free Energies= -234.491185
| Structure | HF/321-G (hartree) | B3LYP/6-31G* (hartree) |
|---|---|---|
| Chair TS | -231.61932001 | -234.61068500 |
| Boat TS | -231.61932127 | -234.60310664 |
| Anti2 (reactant) | -231.69253516 | -234.61170273 |
| Activation Energy, ΔE (kcal/mol) | Chair = 45.9 kcal/mol
Boat = 45.9 kcal/mol |
Chair = 1.11 kcal/mol
Boat = 5.30 kcal/mol |
The activation energy was calculated using the data from each basis set, the top value is the value for the chair conformer and the bottom the value for the boat conformer.The energy values for the 3-21G basis set are very different compared to the energy values calculated by the 6-31G basis set therefore the values calculated from these 2 different calculations can not be compared quantitatively. The lower level basis set showed little difference in the activation energy of both the Chair and boat form whereas the higher level basis set showed that the chair conformer had a lower activation energy then the boat conformation, this fits what is expected by theory.
The Diels Alder Cycloaddition
The Diels Alder reaction is another type of peri cyclic reaction, this time it is a cycloaddition reaction. This shows the same characteristics as the sigmatropic reaction in the fact that the reaction goes by a concerted process with no intermediates.
The reaction involves the concerted formation or cleavage of 2 σ bonds between the termini of two conjugated π systems. 2π systems are involved, one contributing 4π electrons, the other 2π electrons. The total electron count is 6 which means that there are 4n+2 electrons in the thermal reaction and the reaction proceeds via Huckel topology with only suprafacial components and goes through an aromatic transistion state. [1] The reaction also is only allowed to happen by the selection rules if symmetry is conserved throughout the reaction.
[1] In general the HOMO/LUMO of one fragment interacts with the HOMO/LUMO of the other reactant to form two new bonding and anti-bonding MOs. The nodal properties allow one to make predictions according to the following rule: If the HOMO of one reactant can interact with the LUMO of the other reactant then the reaction is allowed.
The HOMO-LUMO can only interact when there is a significant overlap density. If the orbitals have different symmetry properties then no overlap density is possible and the reaction is forbidden.
Cis butadiene optimization
Item Value Threshold Converged?
Maximum Force 0.000100 0.000450 YES
RMS Force 0.000035 0.000300 YES
Maximum Displacement 0.001414 0.001800 YES
RMS Displacement 0.000630 0.001200 YES
Predicted change in Energy=-7.540887D-08
Optimization completed.
-- Stationary point found.
| HUMO | LUMO |
|---|---|
 |

|
| antisymmetric to plane | symmetric to plane |
cis-butadiene has C2v symmetry with its principle axis being the C2 axis running down the center of the butadiene molecule. The σv plane runs parallel to the principle axis through the center of the molecule. The symmetry of the reacting Fronter Molecular Orbitals is shown above. The HOMO orbital is antisymmetric with respect to the σv plane and the LUMO is symmetric with respect to the σv plane.
Computation of the Transition State geometry for Cyclohexene Diels Alder
The transition structure for the reaction between ethylene and butadiene is known to have an envelope type structure where the π systems have maximum overlap
. A bicyclo system was constructed and then edited to make a guess structure for the transition state of the reaction with the terminal carbons of the butadiene fragment being approximately 2.2A from the ethylene fragment. The transition state was optimized using the optmisation to TS(Berny) calculation, the force constant was calculated once and the HF/3-21G method and basis set were used, the frequency was also calculated.
Item Value Threshold Converged?
Maximum Force 0.000027 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000652 0.001800 YES
RMS Displacement 0.000150 0.001200 YES
Predicted change in Energy=-6.921393D-09
Optimization completed.
-- Stationary point found.
The item table confirms that a minimum was found.
Low frequencies --- -610.2216 -2.4676 -0.0003 -0.0002 0.0004 1.5013 Low frequencies --- 1.8941 57.6953 147.1134 ****** 1 imaginary frequencies (negative Signs) ******
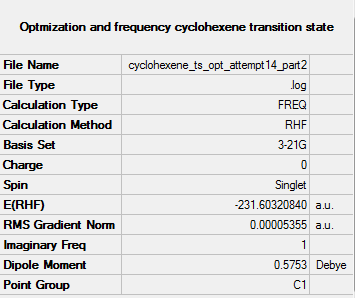
When looking at the gif file for the above optimization, it was seen that I had left too many hydrogens on carbon 2 and carbon 4 of the cis butadiene fragment, therefore the optimization calculation was redone, shown below. [Cyclohexene ts opt freeze part 1] [Cyclohexene ts opt freeze part 2]
Item Value Threshold Converged? Maximum Force 0.000231 0.000450 YES RMS Force 0.000054 0.000300 YES Maximum Displacement 0.001465 0.001800 YES RMS Displacement 0.000559 0.001200 YES Predicted change in Energy=-1.906251D-07 Optimization completed. -- Stationary point found.
The Item table confirms that a minimum was found
Low frequencies --- -818.3091 -1.6233 -0.0004 0.0004 0.0006 4.5508 Low frequencies --- 6.8583 166.7345 284.3552
The vibration frequencies (second line of low frequencies) are all positive confirming that a minimum and not a maximum turning point has been found. There was also one imaginary frequency found at a frequency of -818 cm-1 this corresponds to the bonds which are breaking/being made in the reaction, the imaginary frequency has been visualized below.
The imaginary frequency shows how the reaction proceeds, the ethylene fragment approaches from above or below the ring and attaches at the same side. The bond formation at both terminal carbons happens at the same time which supports the theory that the reaction goes via a pericyclic mechanism.
Typical bond lengths in structure
Typical C-C sp3-sp3 bond length: 1.46 A Typical C-C sp2-sp2 bond length: 1.35 A
The distance between the carbons where bonds are formed and broken is 2.21 A in the optimized transition state the are much larger than either an sp3 or sp2 hybridized carbon carbon bond, showing that the a bond is not fully formed. However the Van der Walls radius of Carbon is 1.70 A, twice this is 3.4A both bond lengths are well within the van der waal radius limit for bonding, therefore there will be orbital overlap between the fragments and there will partial bond formation between the carbon atoms of the 2 fragments in the transition state. The fact that a full bond is not formed is expected as the calculation shows the transition state and not a minimum.
| Imaginary Frequency = -818 cm-1 | Lowest Positive Frequency = 167cm-1 |
|---|---|
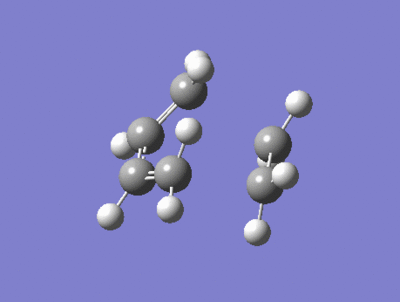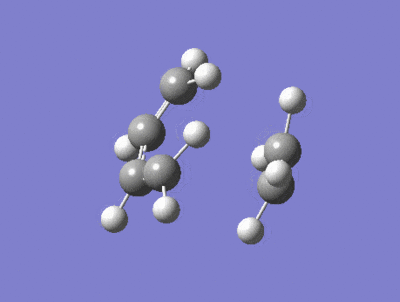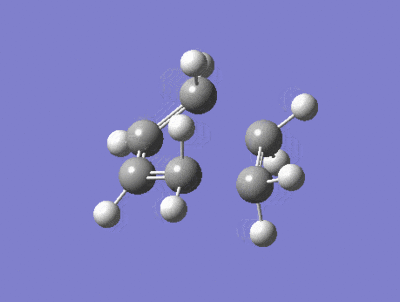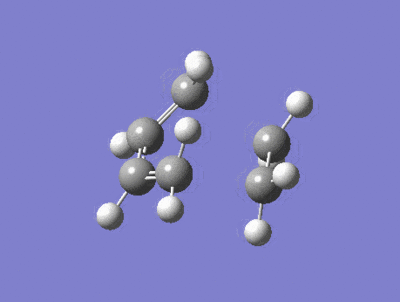
|

|
There is a large difference in the magnitude of the negative imaginary frequency and the lowest positive frequency, the lowest positive frequency shows the vibration of the components in different directions, the ethylene fragment is rotating with asynchronous motion, whether as the Imaginary frequency shows stretching in a synchronous manner which is consistant with a concerted process and peri cyclic reaction.
Tranisition State MO's
| HUMO | LUMO |
|---|---|
 |

|
| antisymmetric to plane | symmetric to plane |
In the ethylene fragment (dienophile) the LUMO is anti symmetric and the HOMO is symmetric, for the butadiene (diene) the HOMO is anti symmetric and the LUMO is symmetric. The Butadienes HOMO combined with the ethene's LUMO as they both have antisymmetric symmetry to form an antisymmetric HOMO in the transition state. The LUMO of of the Butadiene and the HOMO the ethylene combined to give the antibonding LUMO of the transition state as both have symmetric symmetry with respect to the plane.
cyclohexa-1,3-diene reaction with maleic anhydride
In this part of the investigation the regio selectivity of the Diels Alder reaction is investigated. There are 2 possible products formed from the reaction of cyclohexa-1,3-diene reaction with maleic anhydride, the Endo form which is the major, kinetic product and the exo form which is the thermodynamically more stable product and it contains less steric repulsions. The Endo transition state has a lower activation energy accounting for its kinetic favourablity.
Endo transition state optimization
[Endo optimized transtion state]
The Transition state was optimized using the frozen coordinate method and the HF/3-21G method and basis set.
Summary Table for optimization on the Endo transition state
Item Value Threshold Converged?
Maximum Force 0.000059 0.000450 YES RMS Force 0.000010 0.000300 YES Maximum Displacement 0.001623 0.001800 YES RMS Displacement 0.000312 0.001200 YES Predicted change in Energy=-6.047771D-08 Optimization completed. -- Stationary point found.
The Item table confirms a minimum was found.
Low frequencies --- -643.4302 -2.0035 -0.0008 -0.0006 -0.0004 1.5715
Low frequencies --- 2.2893 64.9833 142.0653 ****** 1 imaginary frequencies (negative Signs) ******
There is 1 imaginary frequency corresponding to the bond forming and breaking at -634.4 cm-1.
Exo transition state
The Transition state was optimized using the frozen coordinate method and the HF/3-21G method and basis set.
Item Value Threshold Converged? Maximum Force 0.000063 0.000450 YES RMS Force 0.000013 0.000300 YES Maximum Displacement 0.001537 0.001800 YES RMS Displacement 0.000296 0.001200 YES Predicted change in Energy=-4.122802D-07 Optimization completed. -- Stationary point found.
Low frequencies --- -587.1761 -24.6177 -21.8684 -1.2989 0.0008 0.0011
Low frequencies --- 0.0011 42.0264 129.5490 ****** 1 imaginary frequencies (negative Signs) ******
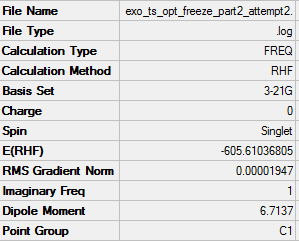
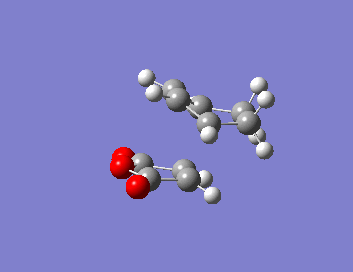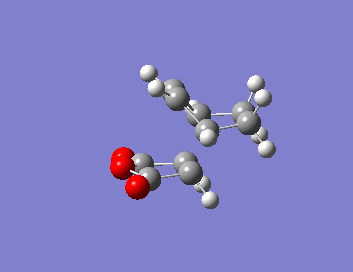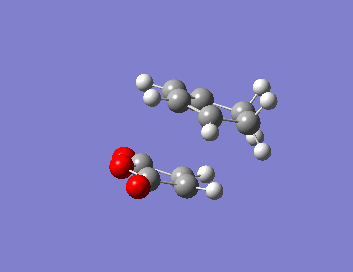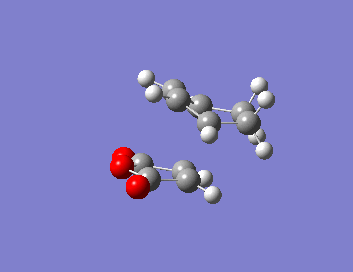
| Isomer | HOMO | LUMO | Energy (au) | Bond Lengths (A) | Through Space Distance (A) |
|---|---|---|---|---|---|
| ENDO | 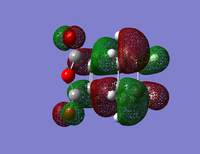 |
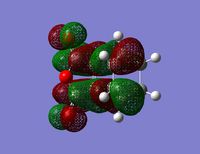 |
-605.61036(805) |  |

|
| EXO |  |
 |
-605.60280(446) | 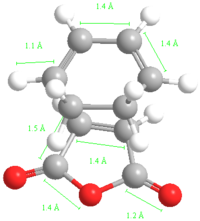 |

|
The energy of the Endo transition state is slightly lower than that of the exo as expected. The length of the single carbon bond in the butadiene fragment is similar to that of the double carbon bond length giving evidence for the aromatic transition state of the reaction.
References
- ↑ 1.0 1.1 1.2 1.3 http://www.ch.ic.ac.uk/local/organic/pericyclic/.
- ↑ 2.0 2.1 2.2 2.3 Cite error: Invalid
<ref>tag; no text was provided for refs namedtutorial - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpaper1 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpaper2 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpaper3 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedbond
Cite error: <ref> tag with name "tutorial" defined in <references> is not used in prior text.
[1] </references> [2] </references>
- ↑ Normal 0 false false false EN-GB X-NONE X-NONE http://pubs.acs.org/doi/pdf/10.1021/ja00111a016 .
- ↑ Normal 0 false false false EN-GB X-NONE X-NONE Normal 0 false false false EN-GB X-NONE X-NONE http://pubs.acs.org/doi/pdf/10.1021/ja00333a024
Cite error: <ref> tag with name "paper3" defined in <references> is not used in prior text.