Rep:Mod:23
Computational Inorganic Chemistry
Week 1 - Gaussian Introduction
Optimisating a molecule of BH3
First Optimisation
BH3 was first optimised using the 3-21G basis set
| File Name | BH3_OPT |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 3-21G |
| Charge | 0 |
| Spin | singlet |
| E(RB3LYP) | -26.46226338 a.u. |
| RMS Gradient Norm | 0.00020672 a.u. |
| Dipole Moment | 0.00 Debye |
| Point Group | D3H |
The Item table shows the optimization ran successfully and an energy minimum was reached for this basis set.
Item Value Threshold Converged?
Maximum Force 0.000413 0.000450 YES
RMS Force 0.000271 0.000300 YES
Maximum Displacement 0.001610 0.001800 YES
RMS Displacement 0.001054 0.001200 YES
Predicted change in Energy=-1.071764D-06
Optimization completed.
-- Stationary point found.
Second Optimisation
The BH3 molecule was optimised for a second time using a larger basis set, 6-31g(d,p)
File:BH3 OPT 631G DP jar110.LOG
| File Name | BH3_OPT_631G_DP |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -26.61532347 a.u. |
| RMS Gradient Norm | 0.00010777 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.00 Debye |
| Point Group | C2V |
Item Value Threshold Converged?
Maximum Force 0.000216 0.000450 YES
RMS Force 0.000141 0.000300 YES
Maximum Displacement 0.000847 0.001800 YES
RMS Displacement 0.000555 0.001200 YES
Predicted change in Energy=-2.943386D-07
Optimization completed.
-- Stationary point found.
The Item table shows the optimisation was successful. The summary table however shows, the molecule having the point group C2V, this is not accurate as the molecule was optimised with the key words nosymm. Upon inspecting bond lengths and angles the molecule still has D3h symmetry which was expected.
| B-H bond length | 1.19 Å |
| H-B-H bond angle | 120° |
TlBr3 Optimisation
A medium level basis set was used carrying out this optimisation. Due to Tl and Br being heavier atoms, in particular Tl. Basis sets for these atoms are not full defined so pseudo potentials are required to carry out the optimisation.
| File Name | tlbr3_opt |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | LANL2DZ |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -91.21812851 a.u. |
| RMS Gradient Norm | 0.00000090 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.00 Debye |
| Point Group | D3H |
The symmetry for this molecule was set at D3H and remained D3H at the end of the optimisation. The energy also reached a minima with a stationary point being found.
Item Value Threshold Converged?
Maximum Force 0.000002 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000022 0.001800 YES
RMS Displacement 0.000014 0.001200 YES
Predicted change in Energy=-6.084107D-11
Optimization completed.
-- Stationary point found.
| Tl-Br Bond Length | 2.65 Å |
| Br-Tl-Br Bond Angle | 120.0° |
A literature value for the Tl-Br Bond length was found to be 2.52Å[1]. This is somewhat shorter than the bond length generated using Gaussian. Considering pseudo potentials were used the bond length generated is not too unrealistic.
The optimised bond angle was 120.0 Å showing the optimised molecule held tight D3H symmetry
BBr3 Optimisation
This optimisation required using a defined basis set for Boron and a pseudo potential for the larger element Bromine.
File:BBR3 OPT 631G DP jar110.LOG
| File Name | BBR3_OPT_631G_DP_jar110 |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | Gen |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -64.43645277 a.u. |
| RMS Gradient Norm | 0.00000383 a.u. |
| Imaginary Freq | |
| Dipole Moment | 0.00 Debye |
| Point Group | CS |
The symmetry group CS reflects the nosymm entered into the calculation. The item table shows an energy minimum was found and the molecule was optimised.
Item Value Threshold Converged?
Maximum Force 0.000008 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000036 0.001800 YES
RMS Displacement 0.000024 0.001200 YES
Predicted change in Energy=-4.094548D-10
Optimization completed.
-- Stationary point found.
| B-Br Bond Length | 1.93Å |
| Br-B-Br Bond Angle | 120.0° |
Comparing Optimisations
| Bond Type | Bond Length |
|---|---|
| B-H | 1.19Å |
| B-Br | 1.93Å |
| Tl-Br | 2.65Å |
A Definition of a bond is very subjective, however broadly speaking it is an attraction between two atoms. More specifically a bond can be thought of as representing the electron density build up which can be defined as the total of all the orbital contribution. Bonding is where there is a build up of electron density in the internuclear region and anti-bonding is where there is a lack of electrons in the inter-nuclear region. Looking at the linear combination of atomic orbitals specifically a bond can be defined as a combination of 2 molecular orbitals with the correct symmetry, alignment and similar energy.
Unsurprisingly B-H has the shortest bond length. Boron and Hydrogen both have valence orbitals low lying in energy which can have strong overlap. Resulting in a less diffuse Molecular Orbital. The B-Br bond length is 0.74Å longer, this is due to size of Br. The valence orbitals for Br are more diffuse and thus the interaction with Boron is not as strong resulting in a weaker longer bond. Considering solely the covalent radii of Br compared to H gives a clear indication the bond length will be longer. The Covalent radii of BR is 120pm compared to 31pm for Hydrogen (approximate values).
Tl-Br has the largest bond length by far. This is because Tl is towards the bottom of group 13 in the periodic table this means that its atomic radii is much bigger and the valence orbitals are much more diffuse.
BH3 Frequency
A frequency calculation was completed on the 6-31g (d,p) optimised BH3 molecule.
File:JONATHANROBSON BH3 FREQ.LOG
Frequencies which have a good basis set should have a first set of low frequencies in the range of ±12. However the below data gives -52.9 this shows the basis set may need to be improved.
Low frequencies --- -52.9351 -47.9181 -47.8765 0.0002 0.0005 0.0011 Low frequencies --- 1162.1937 1212.6476 1212.7080
The second set of low frequencies are all positive however which shows there was no error there in the calculations.
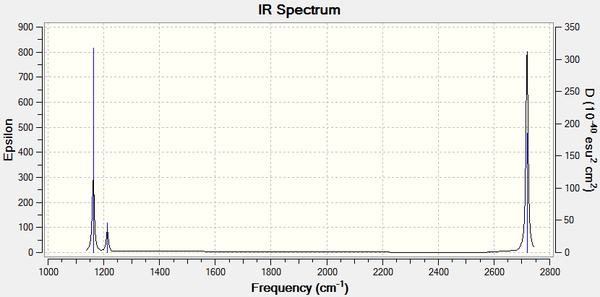
IR Spectrum
There are only 3 peaks in the IR spectrum, where there are 6 vibration modes. The large peak at 1162cm-1 is due to the wagging motion of the BH3 molecule. The next much smaller intensity peak at 1213cm-1is due to both the scissoring and rocking motion which vibrate at the same frequency. The symmetric stretch at 2585cm-1 is not visible due there being no change in dipole moment. The final large peak at 2718 is due to the two antisymmetric stretches. Thus there are only 3 visible peaks on the IR spectrum.
TlBr3 Frequency
The frequency analysis was ran on the optimised TlBr3 molecule. Frequencies below +/- 12 were achieved which shows that the basis set using pseudo potentials was good enough
Low frequencies --- -3.4213 -0.0026 -0.0004 0.0015 3.9367 3.9367 Low frequencies --- 46.4289 46.4292 52.1449
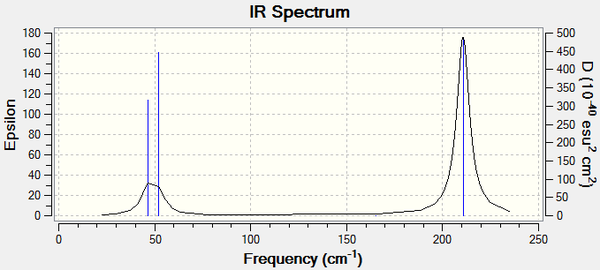
IR Spectrum
The IR Spectrum produced 3 peaks which was not surprising as it shares the same the point group as BH3
Interestingly the intensities for the peaks are different:
Comparing BH3 and TlBr3 vibrations
| No. | Motion | BH3 Frequency (cm-1) | TlBr3 Frequency (cm-1) |
|---|---|---|---|
| 1 | Wagging | 1162 | 52 |
| 2 | Scissoring | 1213 | 46 |
| 3 | Rocking | 1213 | 46 |
| 4 | Symmetric Stretch | 2585 | 165 |
| 5 | Antisymmetric Stretch | 2718 | 210 |
| 6 | Antisymmetric Stretch | 2718 | 210 |
They both have 3 IR peaks which is not surprising due to both having the D3h point group. There are 2 main differences in the IR spectrum; the first is the general frequency of the motions when comparing the molecules. Mass has a great effect on IR frequencies and thus TlBr3 which contains much heavier atoms has very low frequencies compared to BH3. The wagging motion also had the smallest frequency in BH3 whereas it is 3rd in TlBr3. This may also be due to the greater mass in TlBr3.
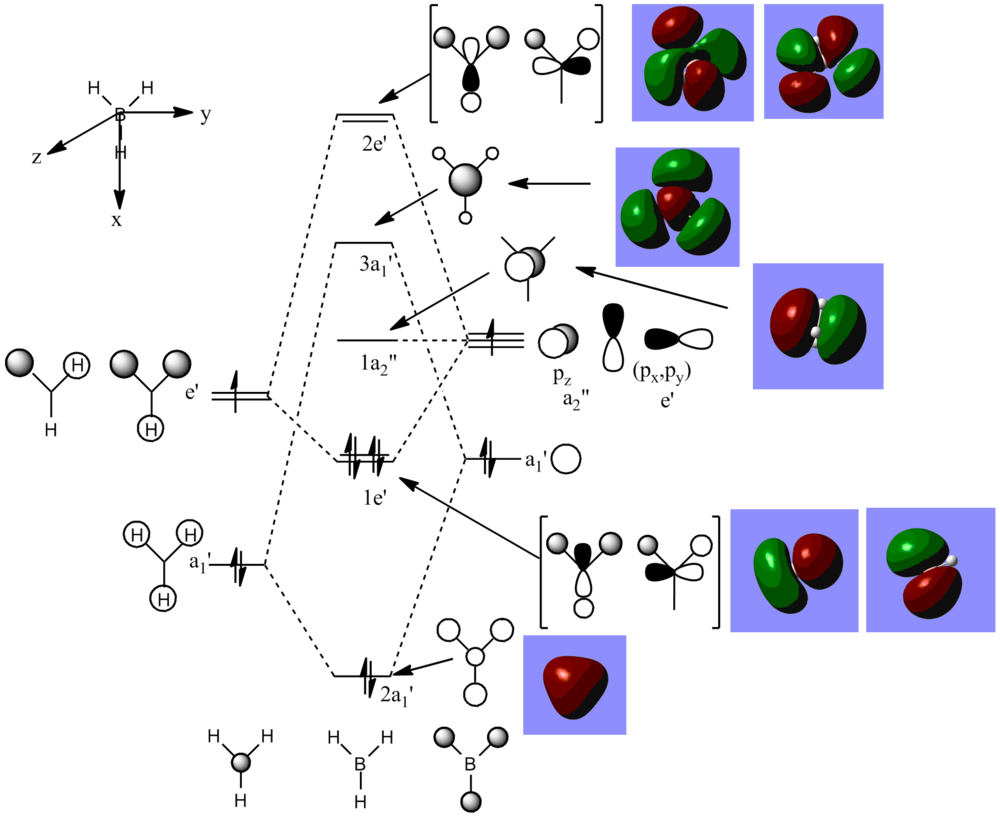
BH3 orbitals
The BH3 MO orbitals was next analysed
There is little difference between the real Molecular Orbitals and the LCAO molecular orbitals, each Molecular Orbital matches well with the corresponding LCAO. The Molecular Orbitals show a more realistic picture combining atomic orbitals into a delocalised system. This shows that MO theory is very useful and can be used in conjunction with calculations well.
NH3 NBO Analysis
NH3 Optimisation
NH3 was optimised using the 6-31g(d,p) basis set
| File Name | logfile(11) |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -56.54794765 a.u. |
| RMS Gradient Norm | 0.00001026 a.u. |
| Dipole Moment | 1.9122 Debye |
| Point Group | C3V |
Item Value Threshold Converged? Maximum Force 0.000019 0.000450 YES RMS Force 0.000012 0.000300 YES Maximum Displacement 0.000042 0.001800 YES RMS Displacement 0.000028 0.001200 YES Predicted change in Energy=-1.075064D-09 Optimization completed. -- Stationary point found.
The optimisation was successful as shown in the item table.
NH3 Frequency
Low frequencies --- -30.6847 -0.0006 0.0010 0.0013 20.2705 28.3113 Low frequencies --- 1089.5554 1694.1245 1694.1864
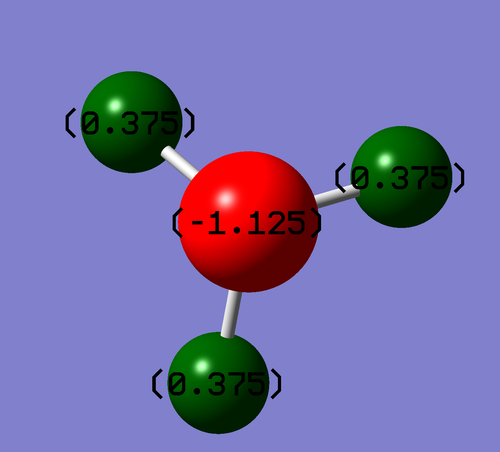
NH3 Population Analysis
Colour Range -1.125 to 1.125
The NBO file showed the expected result. As the most electronegative atom, with a lone pair of electrons the N carries most of the electron density.
Ammonia-Borane
Ammonia-Borane Optimisation
The Ammonia-Borane was optimised using the 6-31g(d,p) basis set
http://hdl.handle.net/10042/20474
| File Name | logfile(13) |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 0 |
| Spin | Singlet |
| E(RB3LYP) | -83.22469032 a.u. |
| RMS Gradient Norm | 0.00005936 a.u. |
| Dipole Moment | 5.56 Debye |
| Point Group | C1 |
Item Value Threshold Converged? Maximum Force 0.000121 0.000450 YES RMS Force 0.000057 0.000300 YES Maximum Displacement 0.000508 0.001800 YES RMS Displacement 0.000294 0.001200 YES Predicted change in Energy=-1.612062D-07 Optimization completed. -- Stationary point found.
This shows the optimisation was successful
Ammonia-Borane Frequency
Low frequencies --- -0.0012 -0.0009 -0.0003 18.5167 23.7903 41.0221 Low frequencies --- 266.2857 632.2325 639.8311
Ammonia-Borane Association Energy
E(NH3)= -56.54794765 a.u. E(BH3)= -26.61532347 a.u. E(NH3BH3)= -83.22469032 a.u.
ΔE=E(NH3BH3)-[E(NH3)+E(BH3)]= -0.0614192 a.u. = -161.3kJmol-1(4sf)
Week 2 - Ionic Liquids: Designer Solvents
Onium Cations
N(CH3)4+
Optimisation
The optimisation was ran with a 6-31g(d,p) basis set
| File Name | logfile(15) |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 1 |
| Spin | Singlet |
| E(RB3LYP) | -214.18127180 a.u. |
| RMS Gradient Norm | 0.00012852 a.u. |
| Dipole Moment | 16.5159 Debye |
| Point Group | C1 |
Item Value Threshold Converged? Maximum Force 0.000237 0.000450 YES RMS Force 0.000078 0.000300 YES Maximum Displacement 0.000952 0.001800 YES RMS Displacement 0.000387 0.001200 YES Predicted change in Energy=-1.043216D-06 Optimization completed. -- Stationary point found.
The optimisation completed successfully.
Frequency
A frequency analysis was then ran.
20882
The low frequency were in an appropriate range.
Low frequencies --- -9.3926 -0.0009 -0.0008 -0.0008 12.4310 16.9229 Low frequencies --- 185.3578 282.9421 289.4049
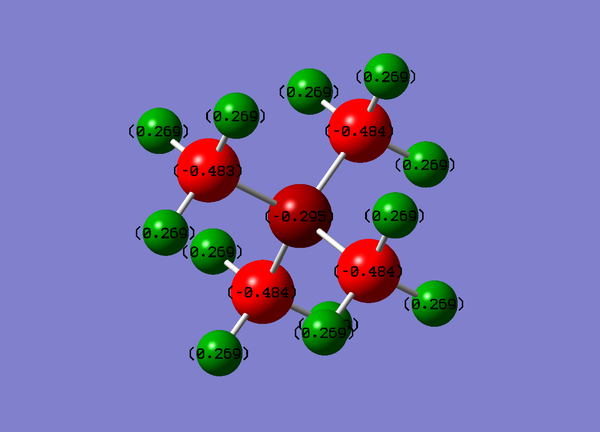
Population Analysis
Colour Range: -0.484 to 0.484
| Atom | Charge |
|---|---|
| N | -0.295 |
| C | -0.484 |
| H | 0.269 |
From these results carbon seems to carry more electron density as N has a lower charge this agrees with the concept that NR4+ carries the formal positive charge.
P(CH3)4+
Optimisation
The optimisation was ran using the 6-31g(d,p) basis set.
| File Name | logfile(17) |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 1 |
| Spin | Singlet |
| E(RB3LYP) | -500.82700419 a.u. |
| RMS Gradient Norm | 0.00000749 a.u. |
| Dipole Moment | 16.5 Debye |
| Point Group | C1 |
Item Value Threshold Converged? Maximum Force 0.000011 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000259 0.001800 YES RMS Displacement 0.000073 0.001200 YES Predicted change in Energy=-5.049072D-09 Optimization completed. -- Stationary point found.
The item table shows the optimisation ran successfully
Frequency
The frequencies were in the appropriate range
Low frequencies --- -18.6580 -7.6000 0.0040 0.0040 0.0042 14.7607 Low frequencies --- 152.5203 182.3425 190.3269
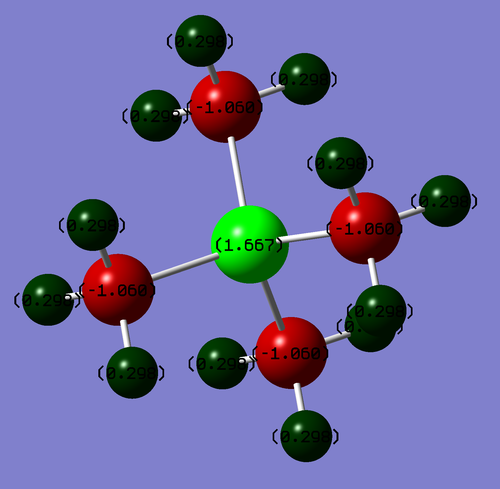
Population Analysis
Colour Range: -1.667 to 1.667
| Atom | Charge |
|---|---|
| P | 1.667 |
| C | -1.060 |
| H | 0.298 |
The Phosphorus has a positive charge so it would make sense that it carries the formal positive charge. The carbon atoms carry a negative charge showing they carry more of the electron density.
S(CH3)3+
Optimisation
The optimisation was ran with the 6-31g(d,p) basis set.
| File Name | logfile(19) |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 1 |
| Spin | Singlet |
| E(RB3LYP) | -517.68327859 a.u. |
| RMS Gradient Norm | 0.00000849 a.u. |
| Dipole Moment | 4.7580 Debye |
| Point Group | C1 |
Item Value Threshold Converged? Maximum Force 0.000015 0.000450 YES RMS Force 0.000007 0.000300 YES Maximum Displacement 0.000490 0.001800 YES RMS Displacement 0.000177 0.001200 YES Predicted change in Energy=-8.895051D-09 Optimization completed. -- Stationary point found.
The item table shows the optimisation ran successfully
Frequency
The frequencies are in a reasonable range.
Low frequencies --- -13.7551 -12.4174 -0.0031 -0.0027 0.0027 22.5117 Low frequencies --- 158.5088 194.3900 198.2748
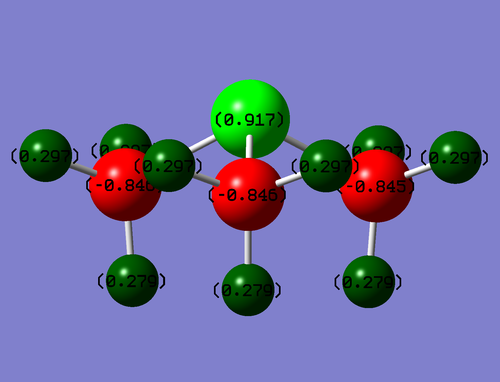
Population Analysis
Colour Range: -0.917 to 0.917
| Atom | Charge |
|---|---|
| S | 0.917 |
| C | -0.846 |
| H 'equatorial' | 0.297 |
| H 'axial' | 0.279 |
S carries the most positive charge so the formal positive charge is likely to reside on that, The C atoms are the most negative suggesting that they carry the most electron density. There is a slight discrepancy between some Hydrogens. 'Axial' Hydrogens in the picture are slightly less positive this may be due to a through space orbital interaction.
NBO Comparative Analysis
| C contribution | X contribution | Charge on C atom | Charge on X atom | |
|---|---|---|---|---|
| C-N | 33.65% | 66.35% | -0.484 | -0.295 |
| C-P | 59.57% | 40.43% | -1.060 | 1.667 |
| C-S | 48.67% | 51.33% | -0.846 | 0.917 |
The contribution in the C-X bond can be rationalised considering electronegativities. N being the most electronegative and having the lowest lying valence AO's contributes the most with 66% of the C-X contribution. S which is the second most electronegative contributes 51% to the bond it has a similar electronegativity to C so they have almost the same contribution. P which has the lowest electronegativity of all contributes the least and C which is more electronegative than P contributes more to the bonding.
This can be related to the charge distribution. Looking first at N; N forms four N-C bonds so the expected charge would be positive however as N contributes 66% towards the bond it draws more of the electron density towards the N atom thus resulting in a negative charge on the N. S which is less electronegative and contributes less to the C-S bond has the expected positive charge. P which contributes the least to the C-P bond has a high charge and carbon has the most negative charge out of all the bonds due to drawing the electron density to it.
Functional Group Influence
[N(CH3)3(CH2OH)]+
Optimisation
The optimisation was ran with a 6-31g(d,p) basis set
| File Name | logfile(21) |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 1 |
| Spin | Singlet |
| E(RB3LYP) | -289.39321646 a.u. |
| RMS Gradient Norm | 0.00003409 a.u. |
| Dipole Moment | 4.9171 Debye |
| Point Group | C1 |
Item Value Threshold Converged? Maximum Force 0.000098 0.000450 YES RMS Force 0.000027 0.000300 YES Maximum Displacement 0.001114 0.001800 YES RMS Displacement 0.000361 0.001200 YES Predicted change in Energy=-1.517196D-07 Optimization completed. -- Stationary point found.
The optimisation was successful as shown in the item table.
Frequency
Low frequencies --- -117.4153 -13.9932 -2.8852 0.0010 0.0010 0.0011 Low frequencies --- 15.7007 129.1708 214.1756
An unexpectedly high frequency of -117 was achieved this may be due to an inadequate basis set
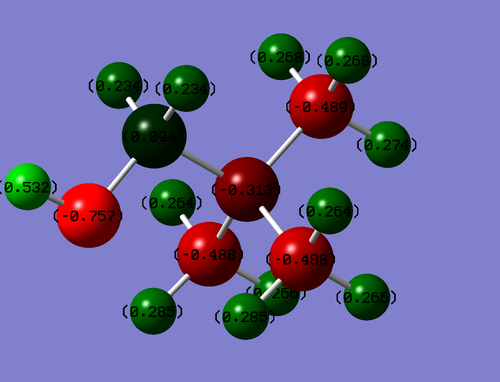
Population Analysis
Colour Range: -0.757 to 0.757
| Atom | Charge |
|---|---|
| N | -0.313 |
| C-OH | 0.094 |
| O | -0.757 |
| O-H | 0.532 |
The carbon bonded to the oxygen has a positive charge due to the electronegative oxygen contributing the most to the C-O bond and thus having the most electron density. The hydrogen bonded to the oxygen also has a positive charge due to the polarising ability of oxygen. If the -OH group had of been bonded to an alkene the lone pair could have been donated into the system. However in this case the lone pair on oxygen is unlikely to be donated into the system as it would result in a formal positive charge on oxygen and a formal negative charge on carbon which is not the most stable arrangement.
[N(CH3)3CN]+
Optimisation
The optimisation was ran using the 6-31g(d,p) basis set
| File Name | logfile(23) |
| File Type | .log |
| Calculation Type | FOPT |
| Calculation Method | RB3LYP |
| Basis Set | 6-31G(d,p) |
| Charge | 1 |
| Spin | Singlet |
| E(RB3LYP) | -306.39375885 a.u. |
| RMS Gradient Norm | 0.00000414 a.u. |
| Dipole Moment | 6.4999 Debye |
| Point Group | C1 |
Item Value Threshold Converged? Maximum Force 0.000013 0.000450 YES RMS Force 0.000003 0.000300 YES Maximum Displacement 0.000915 0.001800 YES RMS Displacement 0.000196 0.001200 YES Predicted change in Energy=-4.853133D-09 Optimization completed. -- Stationary point found.
The optimisation was successful as shown in the item table.
Frequency
Low Frequencies in the given range were achieved
Low frequencies --- -13.3557 -7.2818 -0.0004 0.0008 0.0009 8.6538 Low frequencies --- 91.0529 154.2556 208.3303
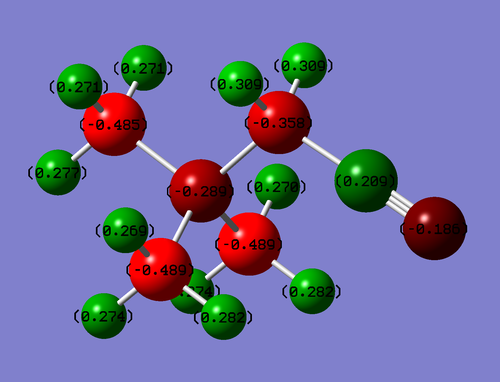
Population Analysis
Colour Range: -0.489 to 0.489
| Atom | Charge |
|---|---|
| N | -0.289 |
| C | -0.358 |
| -CN | 0.209 |
| -CN | -0.186 |
The electron withdrawing effects of CN can be seen. The carbon bonded to the CN group is less negative than all of the other carbons (-0.358 compared to -0.485) this is due to the nitrile withdrawing electron density via induction however it can not contribute back into the system with a lone pair of electron.
HOMO and LUMO Comparison
| Molecule | HOMO | LUMO |
|---|---|---|
| N(CH3)4+ | 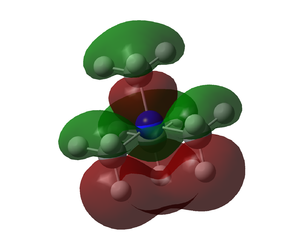 |
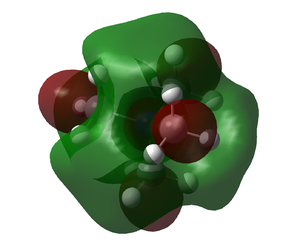
|
| [N(CH3)3(CH2OH)]+ | 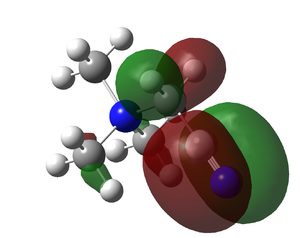 |
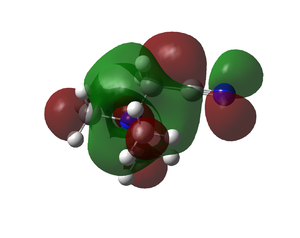
|
| [N(CH3)3CN]+ | 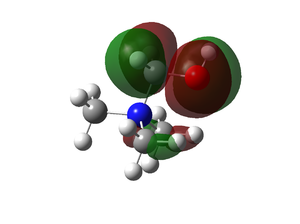 |
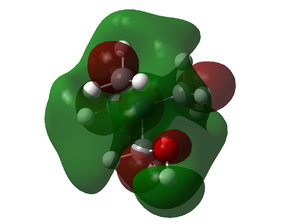
|
| Molecule | HOMO | LUMO |
|---|---|---|
| N(CH3)4+ | -0.57911 a.u. | -0.13312 a.u. |
| [N(CH3)3(CH2OH)]+ | -0.46629 a.u. | -0.11996 a.u. |
| [N(CH3)3CN]+ | -0.50048 a.u. | -0.18187 a.u. |
The HOMO of N(CH3)4+ is unlike the other two which happen to be similar. The other two HOMO orbitals show much less delecalisation with electron density mainly on the -OH, -CN groups respectively. The LUMO's look a lot more similar however there is more dissociation on the N(CH3)4+.
The addition of the -OH group has caused the whole system to be raised in energy. Both the HOMO and LUMO have been raised in energy, HOMO-LUMO gap is smaller however. Even though the HOMO-LUMO gap is smaller the energy of both being raised will cause the molecule to be less reactive as electrons from another molecule are less likely to be donated into the system due to the high energy LUMO. The high energy HOMO may also mean the molecule slightly less stable, this can also be rationalised in the MO as there is a lack of bonding interactions in the Molecular Orbital compared to N(CH3)4+ HOMO.
Interestingly the addition of the -CN group has also raised the energy of the HOMO but the LUMO has been reduced in energy and the gap between the HOMO-LUMO is also smaller. This will result in [N(CH3)3CN]+ being more reactive.
References
<references> [1]
- ↑ 1.0 1.1 Blixit J, Glaser J, Mink J, Persson I,Persson P, Sandstroem M; J. Am. Chem. Soc., 1995, 117 (18), pp 5089–5104. 10.1021/ja00123a011