Rep:Mod:201000CBF
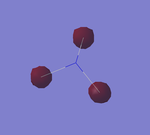
NH3 Optimisation
- Calculation Method : RB3LYP
- Basis Set : 6-31G(d.p)
- Final Energy : -56.55776873 a.u.
- Point Group : C3v
- RMS Gradient : 0.00000485 a.u.
- N-H bond distance : 1.01798 ± 0.001 Å
- H-N-H bond angle : 105.741 ± 1°
The optimisation file is linked to here
Item Section
Item Value Threshold Converged? Maximum Force 0.000004 0.000450 YES RMS Force 0.000004 0.000300 YES Maximum Displacement 0.000072 0.001800 YES RMS Displacement 0.000035 0.001200 YES Predicted change in Energy=-5.986280D-10
Jmol of structure:
optimised molecule |
Table of vibrations and IR intensity
| wavenumber cm-1 | 1090 | 1694 | 1694 | 3461 | 3590 | 3590 |
| symmetry | A1 | E | E | A1 | E | E |
| intensity arbitrary units | 145 | 14 | 14 | 1 | 0 | 0 |
| images |  |
 |
 |
 |
 |

|
Snapshot of the Display Vibrations table
Questions:
- How many modes do you expect from the 3N-6 rule? - 6
- Which modes are degenerate? - The two modes with frequency at 1694 cm-1 and the two with frequency at 3590 cm-1
- Which modes are "bending" vibrations and which are "bond stretch" vibrations?
- 1090 cm-1 : bending
- Two degenerate at 1694 cm-1 : bending
- 3461 cm-1 : bond stretch
- Two degenerate at 3590 cm-1 : bond stretch
- Which mode is highly symmetric? - the mode at 3461 cm-1
- Which mode is known as the "umbrella" mode? - the mode at 1090 cm-1
- How many bands would you expect to see in an experimental spectrum of gaseous ammonia? - two bands; one at 1090 cm-1 and one at 1694 cm-1. The other modes of vibration are highly symmetrical and produce a very small change in dipole moment, giving very small intensities which can't be seen in the IR spectrum.
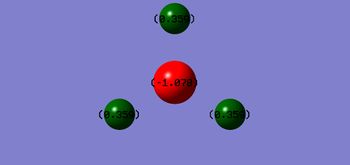
Charge distribution in NH3
As Nitrogen is more electronegative than Hydrogen we would expect Nitrogen to have a partial negative charge and Hydrogen a partial positive charge. This is in accordance with the numbers predicted by Gaussian;
- Charge on N: -1.078
- Charge on H: 0.359
N2 Optimisation
- Calculation Method : RB3LYP
- Basis Set : 6-31G(d.p)
- Final Energy : -109.52412868 a.u.
- Point Group : D∞
- RMS Gradient : 0.02473091 a.u.
- N-N bond distance : 1.10550 ± 0.001 Å
The optimisation file is linked to here
Item Section
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES Predicted change in Energy=-3.400988D-13
Jmol of structure:
optimised molecule |
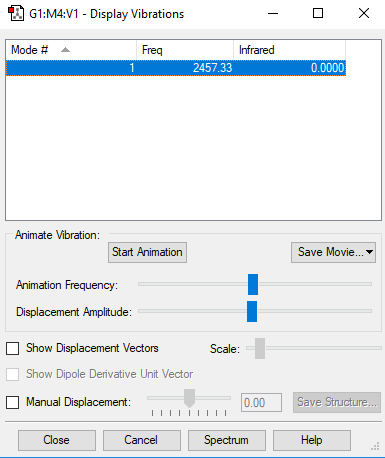
Table of vibrations and IR intensity
| wavenumber cm-1 | 2457 |
| symmetry | SGG |
| intensity arbitrary units | 0 |
Snapshot of the Display Vibrations table
Questions:
- How many modes do you expect from the 3N-5 rule? - 1 stretching mode is present
Charge distribution in N2
As N2 is homonuclear there is no dipole moment and no charge on the individual N atoms.
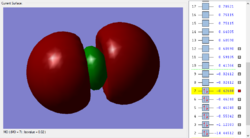
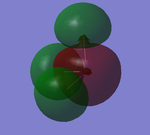
N2 MOs: HOMO and LUMO
H2 Optimisation
- Calculation Method : RB3LYP
- Basis Set : 6-31G(d.p)
- Final Energy : -1.17853936 a.u.
- Point Group : D∞
- RMS Gradient : 0.00000017 a.u.
- H-H bond distance : 0.74279 ± 0.001 Å
The optimisation file is linked to here
Item Section
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES Predicted change in Energy=-1.164079D-13
Jmol of structure:
optimised molecule |
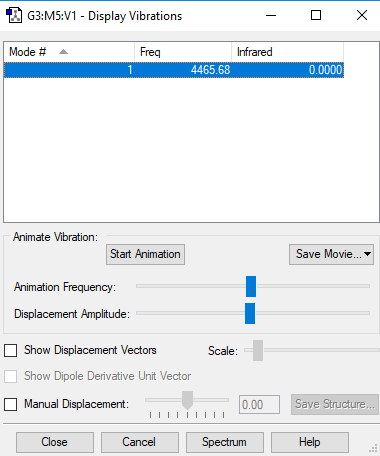
Table of vibrations and IR intensity
| wavenumber cm-1 | 4466 |
| symmetry | SGG |
| intensity arbitrary units | 0 |
Snapshot of the Display Vibrations table
Questions:
- How many modes do you expect from the 3N-5 rule? - 1 stretching mode is present
Charge distribution in H2
As H2 is homonuclear there is no dipole moment and no charge on the individual H atoms.
N2 in a mono-metallic TM complex
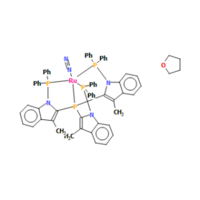
- Unique Identifier: DEKFUX
- N-N bond length in complex: 1.0866 Å
- N-N bond length from Gaussian: 1.10550 ± 0.001 Å
The structure can be found here: [[1]]
The bond length is calculated from the amount of electron density around the atoms. If we have a large electron density region between two positive nuclei, they will be attracted to it shortening the bond.
In our complex, Nitrogen is bound to a Ru atom, this acts as an electron withdrawing group reducing the electron density between the two atoms. This means there is greater repulsion between the two and the bond length is slightly lengthened.
The Haber-Bosch process
E(NH3)= -56.5577687 a.u.
2*E(NH2)= -113.115538 a.u.
E(N2)= -109.5235911 a.u.
E(H2)= -1.1785394 a.u.
3*E(H2)= -3.5356181 a.u.
ΔE= 2*E(NH3) - [E(N2) + 3*E(H2)] = -0.0563288 a.u.
ΔE = -147.8912644 kJ/mol
Ammonia is more stable because the reaction is exothermic therefore its energy is lower than the reactants'.
NF3
- Calculation Method : RB3LYP
- Basis Set : 6-31G(d.p)
- Final Energy : -354.07131058 a.u.
- Point Group : C3v
- RMS Gradient : 0.00010256 a.u.
- N-F bond distance : 1.38404 ± 0.001 Å
- F-N-F bond angle : 101.830 ± 1°
The optimisation file is linked to here
Item Section
Item Value Threshold Converged? Maximum Force 0.000164 0.000450 YES RMS Force 0.000108 0.000300 YES Maximum Displacement 0.000612 0.001800 YES RMS Displacement 0.000296 0.001200 YES Predicted change in Energy=-1.274066D-07
Jmol of structure:
optimised molecule |
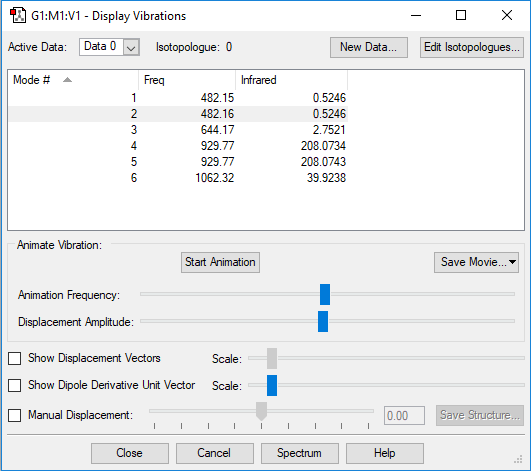
Table of vibrations and IR intensity
| wavenumber cm-1 | 482 | 482 | 644 | 930 | 930 | 1062 |
| symmetry | E | E | A1 | E | E | A1 |
| intensity arbitrary units | 1 | 1 | 3 | 208 | 208 | 40 |
| images |  |
 |
 |
 |
 |

|
Snapshot of the Display Vibrations table
Questions:
- How many modes do you expect from the 3N-6 rule? - 6
- Which modes are degenerate? - The two modes with frequency at 482 cm-1 and the two with frequency at 930 cm-1
- Which modes are "bending" vibrations and which are "bond stretch" vibrations?
- Two degenerate at 482 cm-1 : bending
- 644 cm-1 : bending
- Two degenerate at 930 cm-1 : bending
- 1062 cm-1 : bond stretching
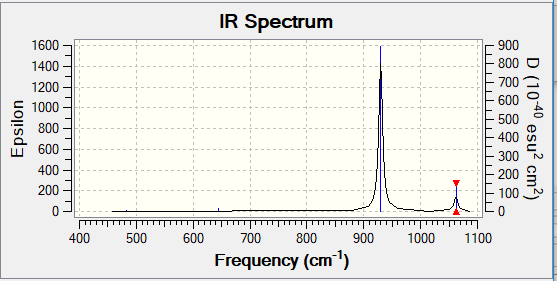
- How many bands would you expect to see in an experimental spectrum of NF3? - two bands; one at 930 cm-1 and one at 1062 cm-1. The other modes of vibration produce a very small change in dipole moment, giving very small intensities which can't be seen in the IR spectrum.
This is the IR spectrum predicted by Gaussian, which clearly shows 2 bands.
Charge distribution in NH3
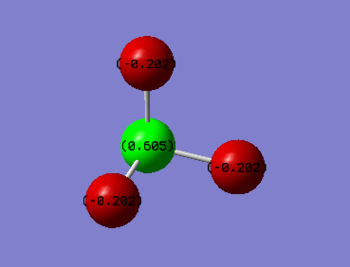
As Fluorine is more electronegative than Nitrogen so, we would expect Fluorine to have a partial negative charge and Nitrogen a partial positive charge. This is in accordance with the numbers predicted by Gaussian;
- Charge on N: 0.605
- Charge on F: -0.202
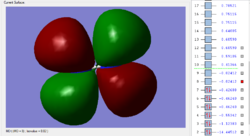
MO orbitals in NF3
- HOMO ENERGY: -0.35162 a.u.
- LUMO ENERGY: 0.01947 a.u.