Rep:Mod:200529ypinorganicproject
Mini Project - "Fuels for the Future"
This mini project uses Gausian/GaussView in order to analyse the use of ammonia borane as an alternative, hydrogen storing fuel for motor vehicles. Ammonia broane will be compared computationally with it's organic analogue, ethane.
Geometry of Ammonia Borane
First year chemists know that ethane is found in the staggered geometry, due to this conformation being energertically more stable. The two different geometries (eclipsed and staggered) of ammonia borane were constructed in ChemBio Ultra and saved as Guassian input files. A DFT based B3LYP calculation with a 3-21G basis set was employed to minimise the energy of both the conformers, to help determine the most energetically favourable. There was an initial problem.
Borane acts as a Lewis acid, accepting electrons into the empty p-orbital on the boron atom. In the formation of ammonia borane, the lone pair in the sp3 orbital of ammonia (a Lewis base) is donated into this empty p-orbital on boron. The construction of this molecule in ChemBio Ultra does not seem to regard this interaction being the reason for the N-B bond, and still includes the lone pair on N in the final structure, shown below in both the staggered and eclipsed conformers.
| Eclipsed NH3BH3 | Staggered NH3BH3 |
|---|---|
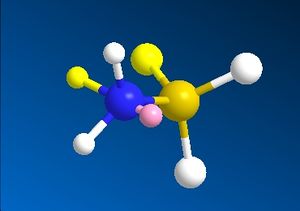 |
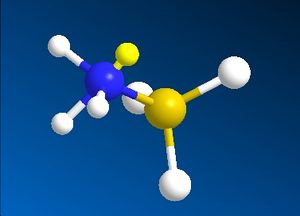 |
This lone pair appeared to effect the geometry of the molecule, giving 5 valent nitrogen! As such, minimisation calculations were carried out on molecules with formal charges on, to account for the transfer of electrons. This was done purely to try and "trick" ChemBio Ultra into aknowledging the lone pair on N was now involved in the N-B bond, if there is no, or little difference in the output from Guassian I will disregard the "charged" species since this is an incorrect description of bonding in ammonia borane. The results from the charged and non charged species are displayed below for comparison.
| Uncharged | Charged | |
|---|---|---|
| Staggered |  |
 |
| Eclipsed |  |
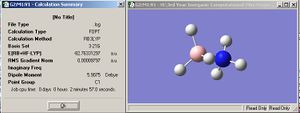 |
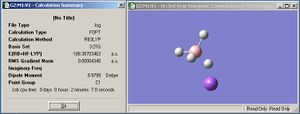
Not surprisingly, the lowest energy conformer (in both the charged and uncharged cases) appears to be the staggered one. In both cases, the difference in energy (and hence the energy barrier to rotation about the N-B bond) is around 7.5kJmol-1(7.5513 and 7.5237kJmol-1 for the charged and uncharged models respectively). The values of the "charged" and "uncharged" species are close enough that we can disregard the incorrect description of bonding in NH3BH3
A similar calculation to that above was applied to ethane, to compare the energy of free rotation about the N-B bond with that about the C-C bond.
| ΔEeclipsed-staggered C-C kJ.mol-1 | ΔEeclipsed-staggered N-B kJ.mol-1 |
|---|---|
| 11.3402 | 7.5237 |
The lower energy difference between the staggered and eclipsed conformers of ammonia borane indicates that this must freely rotate at a lower energy than C-C bonds.
A Note on Bonding in Ammonia Borane

As previously mentioned, the N-B bond is formed via a Lewis acid-base reaction in which the sp3 lone pair on the ammonia fills the empty pz orbial on the boron. In order to investigate this, the table below displays N-H and B-H bond lengths for ammonia borane and for ammonia and borane for comparison.
| Bond | Ammo | Borane | Ammonia Borane |
|---|---|---|---|
| N-H /Å | 1.02216 | - | 1.02761 |
| B-H /Å | - | 1.19453 | 1.21191 |
The N-B bond distance was measured at 1.68505/Å. This initially presents a worry since the wikipedia entry for N-B length cites1 a bond length of 1.58(2). The cited bond length on Wikipedia is taken form the solid phase (standard conditions), however when in the gas phase we can expect this length to be much longer. Since Gaussview calculated the optimisation in geometry for this single molecule, it will have calculated the gas phase optimisation. The paper "Ammonia–borane: the hydrogen source par excellence?", published by Frances H. Stephens et al.2 states the gas phase B-N bond length at 1.6722 Å, the results are in good agreement with cited literature.
References And Literature
- http://en.wikipedia.org/wiki/Ammonia_borane
- Frances H. Stephens, Vincent Pons, R. Tom Baker "Ammonia–borane: the hydrogen source par excellence?" Dalton Transactions, 2007, pp. 2613-2626,DOI:10.1039/b703053c
Relative Stability of Ammonia Borane and the Reactants Used in it's Production
| Ammonia Borane | Ammonium Chloride | Sodium Borohydride |
|---|---|---|
 |
 |
 |
Ammonia Borane in the Solid State
The melting point of ammonia borane is 1100C (1), this is much higher than that quoted for ethane, -1720C 2. While there was no time to carry out calculations, literature provides an explanation for this observation.
Wim T. Klooster et al. provide an explanation for this in their paper3 in which they study the nature of dihydrogen bonds between N-H and H-B. They state that the dihydrogen bond between N-Hδ+ and δ-H-B in a generic NHxBHx is between 1.7-2.2Å, we can therefore predict that the dihydrogen bond lengths in the modelled solid state ammonia borane will be in this range. Further more, a small description of a dihydrogen bond is provided by the authors. Essentially the dihydrogen bond is an interaction between the δ+ proton on the nitrogen and a δ- hydride, located on the boron. The authors make a change saying that the interaction "may be better thought of as an interaction between the NH proton and the BH bond as a whole"3. This overall interaction leads to a bend in the B-H~~H bond. After carrying out a minimisation on the "solid state" ammonia borane, one could measure this angle and compare with the results found within the literature.
References and Literature
- http://www.ch.ic.ac.uk/wiki/index.php/Mod:inorganic
- http://www.sigmaaldrich.com/catalog/search/ProductDetail/FLUKA/00582
- Klooster, W.T. (1999), "Study of the N-H...H-B Dihydrogen Bond Including the Crystal Structure of BH3NH3 by Neutron Diffraction", Journal of the American Chemical Society 121: pp. 6337–6343, DOI:10.1021/ja9825332
