Rep:Mod:200529yp
The basic techniques of molecular mechanics and semi-empirical molecular orbital methods for structural and spectroscopic evaluations
This computational lab course makes use of the ChemBio3D Ultra modelling programme in order to infer information of both thermodynamic and kinetic nature. The programme is also used to derive molecular orbitals of the molecules tested.
The Hydrogenation of Cyclopentadiene Dimer
The dimerisation of cyclopentadiene proceeds predominantly via an endo Diels-Alder mechanism. ChemBio3D Ultra was used to determine the cause of this selectivity. The two possible dimers of cyclopentadiene, shown to the right, were drawn and a minimisation of geometry and energy was carried out. The results are shown in the table.
| Cyclopentadiene dimer formed by Endo Attack | Cyclopentadiene dimer formed by Exo attack | ||||||
|---|---|---|---|---|---|---|---|
|
| ||||||
| Minimum Energy: 31.8834 kCal/mol | Minimum Energy: 34.0153 kCal/mol |
The lower energy shown by the endo formed dimer indicates this to be the most thermodynamically stable. Since the endo dimer is formed specifically over the exo dimer we can conclude that the first part of this reaction scheme is thermodynamically controlled.
Hydrogenation of the endo product yields two posibilities (shown below). We are told one of these possibilities is produced first and only after some length of time does the dihydrogenated product form, this suggests a kinetically controlled reaction in which one C=C double bond is hydrogenated much more quickly than the other one.
| Hydrogenation Product 1 | Hydrogenation Product 2 | ||||||
|---|---|---|---|---|---|---|---|
|
| ||||||
| Minimum Energy: 35.9337 kcal/mol | Minimum Energy: 31.1539 kcal/mol |
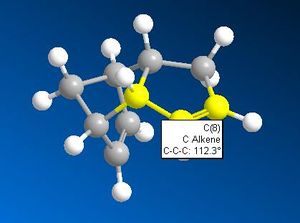
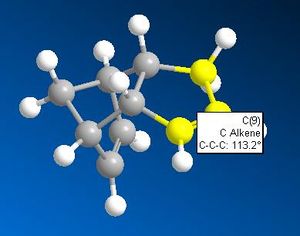
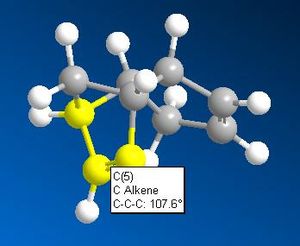
The above table clearly indicates that the product 2 is the most thermodynamically stable product. This does not necessarily make it the first prevailent product however. Analysis of the alkene-cis- carbon bond angles, along with the inter atomic distance between the two cis- carbons of each double bond offers an explanation for the most kinetically favoured product.
| Product 1 | Product 2 |
|---|---|
 |
 |
 |
 |
 |
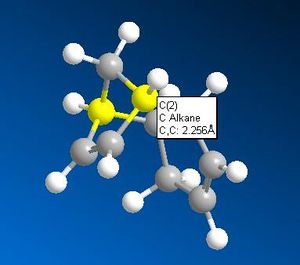 |
Both the smaller cis-carbon distances and the smaller cis-carbon alkene angle of the double bond that breaks to form product 2 indicates this bond to be more strained and therefore weaker than the one that breaks to form product 1. This weaker C=C bond facilitates the electrophilic addition reaction of hydrogen to form product 2 first. The addition of hydrogen across a double bond is irreversible due to the high C-H bond strength. The irreversibility of the hydrogenation reaction accounts for the observation that with enough time, a dihyrogenated product is formed; the other, less strained C=C double bond will eventually be hydrogenated.
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue)
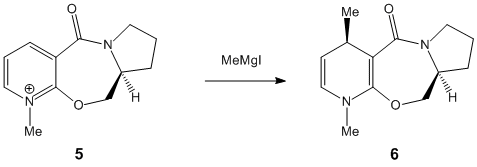
This computational experiment looks at dihedral angles rather than energy in order to help explain the stereocontrol exhibited in the reaction scheme shown to the right.
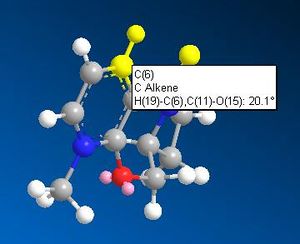
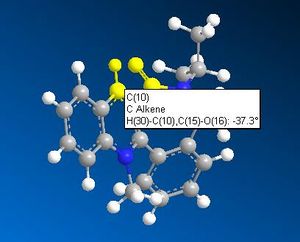
ChemBio3D was used to sketch an initial "guess" conformation of the starting product (5) and an MM2 minimisation of energy was carried out. The 3D model, along with that of an analogue and the dihedral angles between the carbonyl and the aromatic ring, are shown in the table below.
|
| ||||||
|---|---|---|---|---|---|---|---|
 |
 |
The stereochemical control of the reaction 5-->6 is explained with the help of the literature cited below1 which suggests the addition of the methyl to the aromatic system proceeds via an initial coordination between the carbonyl oxygen and the magnesium of the Grignard. A look at the 3D render of NAD displays the carbonyl-aromatic dihedral angle at 20.10 above the aromatic (with respect to the hydrogen on the ring joining carbon). Since the Grignard coordinates to the oxygen above the aromatic ring, the methyl group can only attack the aromatic ring from above. This gives product 6.
The carbonyl group has inverted stereochemistry to the above example, in that it lies below the aromatic ring. Obviously we can not use the same "initial coordination" argument as above since this would result in the adduct pointing down with respect to the chiral carbon's hydrogen. A simple steric argument could initially be used to explain the origin of the steric control exhibited.
The NHPh group is bulky and probably experiences steric repulsion from the large iso-propyl group from the N-substituent in the 7-membered ring. Leleu et al.2 quoted that the size of the attacking electrophile greatly changes the stereoselectivity of a reaction very similar to that shown above. They found that an attacking BH3 unit adds in the same plane, relative to the aromatic ring, as the carbonyl group. After increasing the size of the attacking group to an NaBH4 unit, the major product was found to have opposite stereochemistry to that of the carbonyl oxygen (which pointed down with respect to the ring), which they attributed to the greater steric bulk of NaBH4. This could help explain the observation in the above reaction.
What appears to be the greatest steric interaction in this reaction is that between the carbonyl oxygen and the hydrogen on the isopropyl group, both of which are 1,4 to one another. This is an example of a 1,4 Van der Waals interaction. As can be seen from the jMols below, the 1,4 interaction is reduced in the isomer which shows "up" stereo chemistry with respect to the added NHPh group; this is becasue the hydrogen responsible for this interaction points away from the carbonyl oxygen (the atoms responsible for this interaction are shown in the below table as image files). For the isomer (which isn't formed) that shows "down" stereochemistry with respect to the added amine, this hydrogen points towards the oxygen, increasing the unfavourable, repulsive 1,4 Van der Waals interaction. We can therefore say this reaction is probably thermodynamically controlled.
| Product showing correct stereochemistry | Potential product | ||||
|---|---|---|---|---|---|
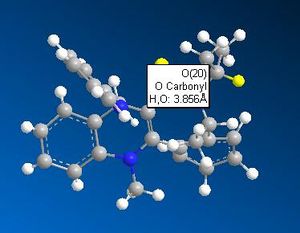 |
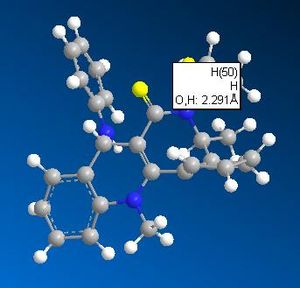 |
References and Literature
- A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838. DOI:10.1021/jo00356a016
- Leleu, Stephane; Papamicael, Cyril; Marsais, Francis; Dupas, Georges; Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928. DOI:10.1016/j.tetasy.2004.11.004 , DOI:10.1016/j.tetasy.2004.11.004
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
| "Carbonyl Down" | "Carbonyl Up" | ||||
|---|---|---|---|---|---|
| Total Energy: 44.2972 kcal/mol | Total Energy: 54.3812 kcal/mol |
After carrying out an MM2 minimisation calculation on both of the possible taxol intermediates shown in the table above, the most stable intermediate was found to be the one in which the C=O bond points in the opposite direction to the two hydrogens on the ring meeting carbons. Since this is the most stable intermediate, given enough time, the reaction pot would be filled with this thermodynamic product. This was first noted by S. W. Elmore and L. Paquette1, who explained the instability of the "carbonyl up" compound in terms of an energetically unfavourable twist-boat conformation of the adjoining 6 membered ring. This is easy to see in the jmol above. The compound in which the carbonyl points down however locks the 6 membered ring into a comfortable chair conformation, being more energetically favourable. To test this idea I modified the twist-boat structure of the cyclohexane ring, to see if I could lock it into a chair conformation. Results are as below.
| Total Energy: 54.3812 kcal/mol | Total Energy: 48.8908 kcal/mol |
Since the energy of the chair cyclohexane is lower, this suggests that infact both the chair and twist-boat conformations exist and actually, the chair cyclohexane predominates. The "down carbonyl" compund is still more stable (by about 4.5 kCal/mol) than the "up carbonyl" compound, so will still be the resultant isomer on standing.
References and Literature
- S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
How one Might Induce Room Temperature Hydrolysis of a Peptide
ChemBio3D was used to help determine the reason for the following experimental result. It is seen that the peptide with the hydroxy group trans to the two ring meeting hydrogens reacts intramolecularly much quicker than its stereoisomer in which the hydroxy group is cis to ring meeting hydrogens.
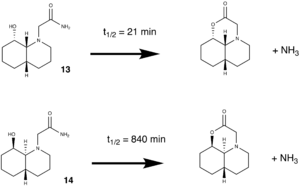
In total four molecules were constructed and an MM2 energy and geometry minimisation was carried out on each. The results are tabulated beolw:
|
|
|
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Energy: 24.5709 kcal/mol | Total Energy: 21.4199 kcal/mol | Total Energy: 27.6002 kcal/mol | Total Energy: 16.4467 kcal/mol | ||||||||||||
| Amine-Hydroxy Distance: 3.125Å | Amine-Hydroxy Distance: 2.587Å | Amine-Hydroxy Distance: 4.623Å | Amine-Hydroxy Distance: 5.182Å |
The above results can be used to explain the difference in reaction rates between the two isomers. Isomer 1 reacts much more quickly than isomer 2. A look at the distances between the two reacting groups (those are the amine and the hydroxy groups) can help explain the quicker reaction rate shown by isomer 1. The 2 shortest amine-hydroxy distances occur in both conformers of isomer 1. This reaction is facilitated by an initial hydrogen bond interaction between a hydrogen on the amine and the oxygen of the hydroxy group, and therefore the closer these groups are together, the more easily the reaction takes place. These results are in line with those cited in the literature1 which states that "the ethylamido group, not surprisingly, prefers to be in the equatorial position (favored over axial by ... 3.1 kcal/mol)" This is handy since also in the equatorial position the Amine hydroxy group distance is the closest possible, the molecule does not need to overcome any large energy barrier before the hydrolysis reaction can take place.
Isomer 2 however has a very much more stable axial isomer, and while these results are not the same as those cited in the above quoted literature, they still follow the same trend. This is an importnat observation and we can state that in order for the hydrolysis reaction to take place, the molecule must overcome a massive energy barrier in order to orientate the hydroxy and amine groups close enough together to experience a hydrogen bonding type interaction. Since hydrolysis involves the driving off of water, the reaction effectively irreversible: this accounts for the stated observation that eventually isomer 2 does hydrolyse. Given enough time, enough moelecules overcome the high energy barrier and hydrolysis occurs.
In summary, the faster reaction rate for the intramolecular hydrolysis of isomer 1 relative to isomer 2 is accounted for by considering the 3D shape of each isomer's most stable conformation. Isomer 1's most stable conformer positions the reacting groups close to each other, speeding up the reaction; isomer 2's most stable conformer has the reacting groups very far apart and must therefore overcome a large energy barrier in order to react.
Literature and References
- M. Fernandes, F. Fache, M. Rosen, P.-L. Nguyen, and D. E. Hansen, 'Rapid Cleavage of Unactivated, Unstrained Amide Bonds at Neutral pH', J. Org. Chem., 2008, 73, 6413–6416 ASAP: DOI:10.1021/jo800706y
Modelling Using Semi-empirical Molecular Orbital Theory
The next section sees the use of a more empirical method than the purely mechanical minimisation, MM2. By considering the positions of all electrons in the system, we get a more accurate description of the molecule under study. A PM3 valence-electron self-consistent-field MO method is employed to construct MOs of the molecule shown in the section below.
Regioselective Addition of Dichlorocarbene
As stated above a PM3 self-consistent-field MO method was employed (using the ChemBioUltra3D program)in order to construct MOs of the molecule below. 5 MOs are also shown below, ranging from the HOMO-1 to the LUMO+2, this will help to rationalise the observation that the endo C=C double bond (wrt the Cl-C bond on the cyclopropene) is more sescepetible to electrophilic attack than the exo double bond, this was reported by B. Halton, R. Boese and H. S. Rzepa1.
| MO | 
|
|---|---|
| HOMO-1 | 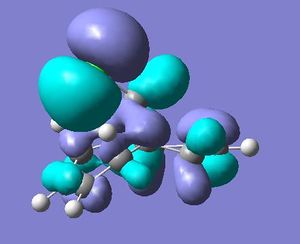 |
| HOMO | 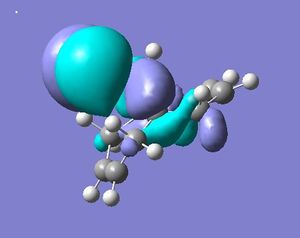 |
| LUMO | 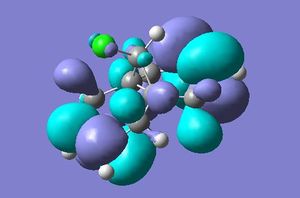 |
| LUMO+1 | 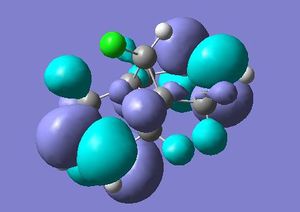 |
| LUMO+2 | 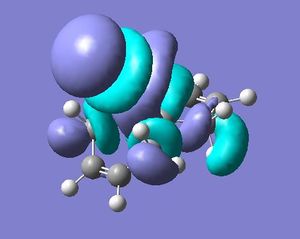 |
The reference below1 is very helpful in explaining the orbital interactions responsible for the attack of the endo C=C bond over attack of the exo C=C bond. The σ*Cl-C (LUMO+2) and exo-π (HOMO-1) orbitals are sufficiently close to allow electron donation from the exo-π system. This reduces electron density from the exo-C=C double bond and therefore reduces it's attractiveness to electrophiles. The literature reports a difference in the distances between the bridging carbon and either of the two alkene carbons in either of the π systems. If this σ*Cl-C-exo-π interaction takes place, the distance between bridging carbon and alkene carbon will be less in the exo case1.
| π System | Bridging Carbon-π System Distance |
|---|---|
| Endo | 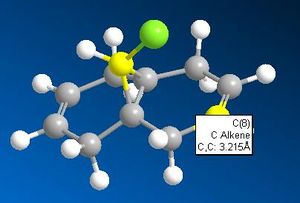 |
| Exo | 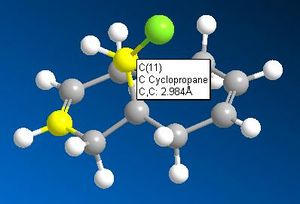 |
While these results don't quite fit those cited in the literature1, they are very close, there being about a 0.3Å decrease in distance for the exo-π system than for the endo. This indicates that this interaction is taking place, rendering the exo double bond less nucleophilic than the endo double bond. Combined with this is the shape of the HOMO orbital, it is seen (above) that there is a high electron density from the chlorine pointing in the direction of the endo- double bond. This adds to the nucleophilic nature of the endo- double bond by increasing the local negative charge about it and therefore the chance of electorhiles attacking.
References And Literature
- B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
Structure Based Mini Project Using DFT Based Molecular Orbital Methods
Assigning Regioisomers In “Click Chemistry”
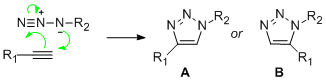 According to the wikipedia page on it, click chemistry "describes chemistry tailored to generate substances quickly and reliably by joining small units together"1. An example of a click chemistry reaction is the formation of a 1,2,3-triazole via a 1,3-dipolar cycloaddition of an azide and an alkyne2. Use of different catalysts results in two different regioisomeric compounds (A or B), for example a copper based catalyst yields mainly 1,4-product, while a ruthenium catalyst results mainly in a 1,5-product. In this section each of the regioisomers are constructed in ChemBio3D, they are then minimised using the SCAN server and an NMR prediction is carried out on them, again using SCAN.Comparison of these two predictive NMRs with the actual NMR obtained (for compound 1 in table 1 of the journal published by Li Zhang et al.3) will tell us which isomer has been formed.
According to the wikipedia page on it, click chemistry "describes chemistry tailored to generate substances quickly and reliably by joining small units together"1. An example of a click chemistry reaction is the formation of a 1,2,3-triazole via a 1,3-dipolar cycloaddition of an azide and an alkyne2. Use of different catalysts results in two different regioisomeric compounds (A or B), for example a copper based catalyst yields mainly 1,4-product, while a ruthenium catalyst results mainly in a 1,5-product. In this section each of the regioisomers are constructed in ChemBio3D, they are then minimised using the SCAN server and an NMR prediction is carried out on them, again using SCAN.Comparison of these two predictive NMRs with the actual NMR obtained (for compound 1 in table 1 of the journal published by Li Zhang et al.3) will tell us which isomer has been formed.
| 1,4 Isomer | 1,5 Isomer | ||||
|---|---|---|---|---|---|
 |
 |
The 13C-NMR data recorded by Li Zhang et al. in CDCl3 gives peaks at chemical shift values δ=51.85, 126.93, 127.22, 128.22, 128.92, 129.08, 129.64, 133.26, 133.34, 135.66, 138.26. When compared with the computed NMR chemical shifts we can deduce which of the isomers was formed in the reaction. The cluster of peaks around 120ppm shifts between ~117-129ppm. Since no peaks are observed between 117ppm and 120ppm, we can conclude that the regioisomer formed in this reaction is that which gives a cluster of signals in the 126-129ppm region of the NMR spectra. This is the 1,5-regioisomer.
References and Literature
- http://en.wikipedia.org/wiki/Click_chemistry (Taken at 10:53 on 31/10/2008)
- http://www.ch.ic.ac.uk/wiki/index.php/Mod:organic#Assigning_regioisomers_in_.22Click_Chemistry.22 (11:02 31/10/2008)
- J. Am. Chem. Soc. 2005, 127, 15998; DOI:10.1021/ja054114s
- https://www.ch.ic.ac.uk/wiki/index.php/Mod:mjs281287 (M. Sheppards Report, from which I used the NMR of the 1,4 regioisomer in "Click Chemistry")