Rep:Mod:1sadia
Module 1: Structure and spectroscopy (Molecular mechanics/Molecular orbital theory)
Sadia Rahman
Introduction
Molecular modeling will be used in the report and it is a computational method used to calculate the different properties of molecules. It avoids trying to solve the Schrödinger equation for the molecule by considering the individual bond properties. This helps to reduce computation time. It uses classical methods and assumes that the contributions are all additive and that they do not interact. The energy is approximated by the summation of diatomic bond stretches, tri-atomic bond angle deformations using Hooke’s law, tetra-atomic bond torsions, van der Waals attractions and repulsions using a simple Lennard-Jones potential and electrostatic attractions. It optimises molecular geometries to a minimum energy and analyses it in terms of bond lengths, angle strain, steric effects and van der Waals contributions.
In the report the Allinger MM2 molecular mechanics module will be used in ChemBio3D, alongside MMFF94 and Gaussian for the DFT methods. The energies calculated from the MM2 method are not thermodynamic quantities and the energies from two different force field cannot be compared. However it can be used for isomer comparison when the two energies calculated use the same force field.
The Hydrogenation of Cyclopentadiene Dimer
Cyclopentadiene dimerises to form the endo (2) dimer over the exo dimer (1). The two dimers are formed by a cycloaddition Diels-Alder reaction. ChemBio 3D was used optimise the geometries of both products using the MM2 force field option. Below is the mechanism along with the transition states. The endo product is formed due to a larger favourable secondary orbital overlap in the endo transition state, which was also stated in literature.[1]
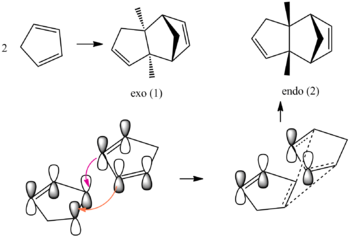
From the calculations it can be seen that the exo product is lower in energy then the endo , by 2.12 kcal mol-1, this is the thermodynamic product but the kinetic product is formed. The reason for kinetic control can be understood by looking at figure 1 and seeing that there is favourable overlap in the endo transition state. The high energy of the endo structure can be rationalised by looking at the interactions of the bridging carbon and the two hydrogen's. In the endo form they are all on the same face and will have an unfavourable interaction, which will not be the case for the exo form.
However to explain the endo product formation despite the greater energy the endo rule can be looked at.[2] It says that the more stable transition state is the one which has the maximum overlap of it molecular orbitals, so its secondary orbital overlap is greater. This is shown in the figure above.
| Total Energy/ kcal mol-1 | |
|---|---|
| Endo | 34.01 |
| Exo | 31.89 |
Hydrogenation of the endo dimer can give two dihydro derivatives: .

It is only after prolonged hydrogenation, the tetrahydro derivative is formed. Again the calculation of the geometries and energies were looked at to decide on the product. The relative contributions from the stretching (str), bending (bnd),torsion (tor), van der Waals (vdw) and hydrogen bonding (H-Bond) energy were computed to look at the relative stability of 3 and 4, as seen in the table below. Looking at the data it suggests that molecule 4 is the thermodynamic product due to lower total energy. Therefore molecule 3 is higher in energy and not the favourable product. The largest energy contribution is from bending, if this is compared for both products then it can be rationalised that 4 is the dominant product. It also has a van der Waals stabilisation energy. The reason for the lower 'bend energy' is due to the position of the double bond and the release of strain in the norborene. Therefore in this case the more stable product is the thermodynamic product.
| Energy contribution/ kcal mol-1 | Molecule 3 | Molecule 4 |
|---|---|---|
| Stretch | 1.25 | 1.13 |
| Bend | 19.06 | 13.02 |
| Stretch-Bend | -0.83 | -0.57 |
| Torsion | 11.17 | 12.40 |
| Non-1,4 VDW | -1.63 | -1.32 |
| 1,4 VDW | 5.80 | 4.44 |
| Dipole/Dipole | 0.16 | 0.14 |
| Total | 34.98 | 29.25 |
Stereochemistry of Nucleophilic additions to a pyridinium ring (NAD+ analogue).
Two reactions of nucleophilic addition to the electron deficient pyridinium ring will be looked at. The first reaction involves the derivative of prolinol (5) which reacts with MeMgI to alkylate the pyridine ring in the 4-position, to give 6. The mechanism for the transformation can be seen below, which shows the absolute stereochemistry.
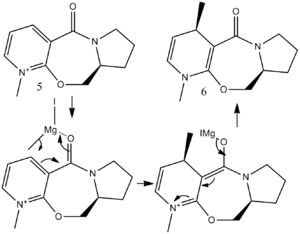
The initial minimum energy of was found after drawing it in ChemBio 3D with the MMFF94 force field and found to be 57.47 kcal/mol with a dihedral angle of about 18oC. The conformation was then altered and the relationship of the dihedral angle and energy looked at. The dihedral angle is between the carbonyl oxygen and carbon, which is alkylated on the adjacent ring, as shown in the diagram below. The carbonyl oxygen and benzyl ring are not fully in plane. The reaction gives a very high selectivity to the product shown. [3] Having a closer look at the mechanism it can be seen that carbonyl has an effect on the Grignard reagent's methyl addition, which gives rise to the stereospecificity. The chelation of the Mg to the oxygen carbonyl also gives rise to the high regio and stereoselectivity.
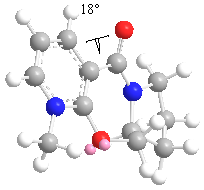
Below is a table summarising some of the variations of the dihedral angle with energy. From theses energies it can be seen that the dihedral angle is a major factor is effecting the minimum energy. This is due to the strain which is cause when the angle is changed. A higher energy system will have a much larger strain so is not favourable. Therefore the methyl group will be above the plane of the ring due to the Mg chelating to the oxygen lone pair, giving the particular stereochemistry shown in the mechanism. In all the low energy conformations the carbonyl group was found to be either planar to the pyridine ring or above the plane of the ring. The higher energy molecules above 100 kcal/mol have the oxygen oriented towards the ring.
| Energy / kcal/mol | Dihedral angle/ 0c |
|---|---|
| 57.47 | 18 |
| 60.80 | 28 |
| 295.43 | 37 |
| 57.53 | -44 |
When the same calculations where tried with MgMeI included and an error message occurred saying 'No atom type was assigned to the selected atom'. This suggests that magnesium is not recognised as an atom type and the parameters are not know. This way around this problem could be to use the MOPAC calculation, define the Magnesium cation parameter and then apply the force field.
In the second example, the pyridinium ring of 7 is made by a reaction with aniline to form 8 which acts to transfer the NHPhenyl group to the electrophilic carbonyl group. Using the example above the origin of the stereocontrol in the formation of 8 can be rationalised by looking at the lowest energy conformations of the reactants. The mechanism for the reaction can be seen below. The products stereochemistry can be explained by looking at the NHPh group, which adds to the same face as the carbonyl. [4]

initial energy was found after drawing it in ChemBio 3D with MMFF94 force field and found to be 113.231 kcal/mol with a diheral angle of about 37.5 oC. However it is not only this angle that needs to be considered but the one between the 6 and 7 carbon rings.
| Energy / kcal/mol | Dihedral angle/ 0c |
|---|---|
| 837.7 | 17 |
| 113.1 | 41 |
| 109.1 | -37 |
| 99.6 | 36 |
| 80.0 | 20 |
 |
 |
 |
 |
Again from the relationship between the dihedral angle and energy the stereochemistry of the product can be rationalised. So the NHR group adds on the opposite face of C=O, so the dihedral angle was found to be small. When the angle was opposite so comparing the energy for 36 and -37o C, it can be seen that the negative angle gave a larger energy, which corresponds to a higher torsion strain and less flexible phenyl group. There is also a steric clash of the butyl group on the amine with the carbonyl oxygen when there is a larger dihedral angle (113.1 kcal/mol) as can be seen from the picture. The dihedral angle was initially positive, this did not explain the stereochemistry. The energy was minimised by changing the orientation of C=O on the ring. The lower energy was found when the carbonyl was on the opposite face. This can be seen form the data and images below. From the data is shows that the energy difference between -37 and 36 o C is not huge but it does show that the dihedral angle explains the stereochemistry of the product. Therefore sterics can explain the products stereochemistry. So the carbonyl group is below the benzene ring and leads to the aniline attacking on the top face to give the product as a masked amide. The energy differences between reactants 7 and 5, is 7 is higher due to the increased ring size and steric clashes.
To improve these calculation the electronic effects need to be taken into account. This could be done by using the MOPAC and PM6 methods, which would allow for the molecular orbital's to be considered so the electronic effects seen.
Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol.
Taxol can be synthesised with the carbonyl group pointing up (9) or down (10): , it is an example of atropisomerisation. They are atropisomers, which are isomers that result from hindered rotation about the single bond, where there is a high steric strain barrier to rotation. The isomer formed will be the one which is more stable. There are two isomers possible one with a twist boat and one with a chair like conformation in the ring. The MM2 force field will be used to decide which isomer is more stable, by looking at its energy and the energy of the hydrogenated forms. The effect of 'hyperstable alkenes' will also be looked at.

| Energy contribution / kcal mol-1 | Molecule 9 | Molecule 10 |
|---|---|---|
| Stretch | 2.39 | 2.59 |
| Bend | 13.52 | 11.93 |
| Stretch-Bend | 0.34 | 0.41 |
| Torsion | 21.08 | 22.16 |
| Non-1,4 VDW | -0.77 | -1.05 |
| 1,4 VDW | 13.57 | 13.84 |
| Dipole/Dipole | 0.26 | 0.14 |
| MM2 Total | 50.60 | 50.01 |
| MMFF94 Total | 67.78 | 66.60 |
The table above indicates that molecule 10 is more stable than molecule 9, this is due to the largest difference being in the 'bend energy'. The reason for the stability in molecule 10 over 9 is due to the lower bending energy, also the steric effects are favourable as when the carbonyl is oriented downwards it does not clash with the bridging isopropyl groups. Therefore molecule 10 is the thermodynamic product as stated in literature.[5] The MMFF94 energies calculated are higher than those for the MM2 calculation. They both show the same result which is that molecule 10 is more stable, so molecule 9 will isomerise to 10, under the correct conditions. This is also true if the Bredt's rule is examined. It tells us that having bridgehead alkene's in small rings is not favourable, due to the excess strain caused.[6]
| Energy contribution / kcal mol-1 | Molecule 9 | Molecule 10 |
|---|---|---|
| Stretch | 4.71 | 3.17 |
| Bend | 24.43 | 18.11 |
| Stretch-Bend | 1.20 | 0.68 |
| Torsion | 22.65 | 22.97 |
| Non-1,4 VDW | 2.35 | 1.04 |
| 1,4 VDW | 18.39 | 16.57 |
| Dipole/Dipole | 0.00 | 0.00 |
| MM2 Total | 73.73 | 62.54 |
The alkene's are slow at reacting due to the bridgehead carbon being strained, so to explain this further the molecules were hydrogenated to the alkane's. The energies were minimised as shown in the data above. Theses energies were higher then the energies calculated for the alkene's, this illustrates the slow reactivity. The intermediates are therefore hyperstable alkene's as the alkane's have a high level of strain so its formation is not favoured. This also leads to consider the strain energy, where the strain energy for the alkene's is greater than the alkane's, as the alkene's have positive strain energy. But it can be negative in the case of the hyperstable alkene's. Both molecule 9 and 10 can be illustrated to be hyperstable alkene's by calculating the strain energy below to be negative. It is worked out taking the torsion energy of alkene minus the alkane.
Molecule 9: 50.60 - 73.73 = -23.13 kcal mol-1
Molecule 10: 50.01 - 62.54= -12.53 kcal mol-1
Modelling Using Semi-empirical Molecular Orbital Theory
In the sections above purely mechanical models were looked at. In this section the electronic aspects of reactivity will be illustrated by considering the electrons in the molecule and how they influence bonds and spectroscopic properties.
Regioselective Addition to Dichlorocarbene
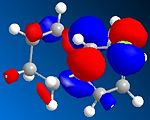
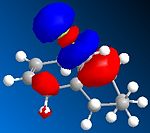
The orbital control of reactivity was looked into with compound 12 with electrophilic reagents such as dichlorocarbene or peracids. The geometry of 12 was predicted and the energy of the orbital's calculated so that they could be seen graphically, as shown in the figures below. ChemBio3D program was used to select the methods and calculate the energy and geometries. MM2 was used to clean the geometry before the electronic method was applied; this generated an energy of 17.90 kcal/mol. Then the MOPAC/PM6 and Gaussian interface was used to minimise the energy and calculate the molecular orbital's and IR spectra of both the di and mono alkenes. The orbitals can be seen below. These were used to help rationalise the electrophilic attack on the dialkene. The monoalkene orbitals were also looked at to analyse this further.
| Molecular Orbital | Dichlorocarbene | Monochlorocarbene |
|---|---|---|
| HOMO -1 |  |
 |
| HOMO | 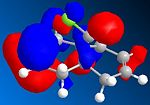 |
 |
| LUMO |  |
 |
| LUMO +1 | 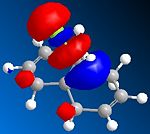 |
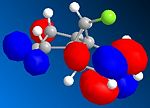 |
| LUMO +2 | 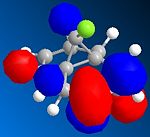 |
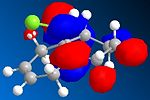 |
Looking at the molecular orbitals the electrophilic attack will occur on the nucleophilic alkene, which will be the one where there is more electron density. The method therefore does distinguish between the two C=C bonds. The HOMO is most reactive to electrophilic attack and the alkene nearest the Cl (syn) has the larger orbital, while the anti alkene orbital is smaller. Therefore the syn alkene is more susceptible to the electrophilic attack; this is therefore the π orbital. Thus the LUMO will be π* orbital for anti and the π* of the syn alkene is the LUMO +2. There is also a secondary overlap between the electron density of the alkene and chlorine. The HOMO-1 is the anti alkenes π orbital and it is set up in an anti-periplanar relationship with the C-Cl σ* orbital which is the LUMO +2. The energies of the orbitals determine the reactivity of the molecule, so the energies of the orbitals can help to understand the regioselectivity.
Now moving onto the monoalkene the orbitals differ in the LUMO where the π* is no longer the LUMO +2 but the LUMO +1 which indicates that it is more nucleophilic as the orbitals energy is lower. It willtherefore be more likely to be attacked by an electrophile. The molecular orbitals are therefore a very important tool is looking at reactivity and rationalising selectivity.
The vibrational energies were generated by using the B3LYP/6-31G (d,p) Gaussian geometry optimization. The frequency calculations were look at to see the influences of the C-Cl bond on the vibrational frequencies of the molecule. The C-Cl stretching frequencies and two C=C stretches for the diene and the single C=C stretch for the monohydrogenated derivative were found and are shown in the table below.
| Frequencies/ cm-1 | C-Cl | C=C |
|---|---|---|
| Dichlorocarbene [7] | 770.8 | 1757.4 (syn),1737.0 (anti) |
| Monochlorocarbene [8] | 775.0 | 1758.1 (syn) |
The IR data shows that the syn frequency is larger than the anti , this is in agreement with the molecular orbital data and the idea that the two alkene bonds are different, as this bond is stronger than the anti one. This indicates that there is stabilisation due to the Cl orbital. The C-Cl bond is weaker due to having a lower vibrational frequency and lower bond order, in the dialkene then in the monoalkene so both the alkene bonds affect it. The syn bond frequencies do not change, so the interaction of this bond has not been effected. When the bond is removed the C-Cl bond stretch increases so there is stronger bond due to the interaction between the double bond and C-Cl antibonding orbital not existing so not weakening it, as mention in literature.[9]
Structure based Mini Project using DFT-based Molecular orbital methods
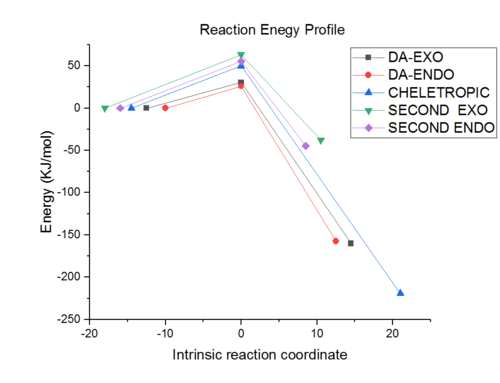
The reaction chosen for the mini project is the "Direct Stereoselective Diels-Alder Synthesis of Benzocantharidin". [10] The reaction involves cycloaddition of isobenzofuran and dimethylmaleic anhydride under reflux of xylene. It gives benzocantharidin in a 95 % yield and 5% isobenzocantharidin. Benzocantharidin is the exo stereosiomer and isobenzocantharidin the endo one. The Diel-Alder reaction was shown to be reversible, which accounts for the high stereoselectivity in favour of the exo isomer. Below are some of the reaction steps considered in the synthesis of the product.
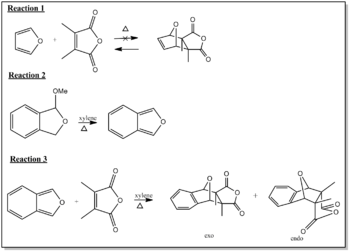
Reaction 1: Diels-Alder reaction of furan and dimethylmaleic anhydride followed by hydrogenation gives cantharidin, however the retro-Diels-Alder reaction can only be achieved to give deoxycantharidin, so it cannot be used as a starting material to produce Benzocantharidin. Therefore a more complex route it taken.
Reaction 2: Here a Diels-Alder reaction is initiated by two circuitous routes to cantharidin to give a stereospecific product. The use of modified furan gives rise to the product to be used in reaction 3 as a starting material which reaction 1 could not provide. The cantharidin skeleton is formed even though the modified furan has electronic and steric deficiencies, to give Isobenzofuran. It is formed by the elimination of methanol from l,3-dihydro-l-methoxyisobenzofuran with heating and excess xylene reflux.
Reaction 3: The stereoselective Diels-Alder Reaction can now take place with isobenzofuran and dimethylmaleic anhydride under heat and reflux of xylene. The exo/endo stereoselectivity of exo (Benzocantharidin) and endo (Isobenzocantharidin) isomers ca. 95/5 in favour of the desired exo isomer. The mechanism can be seen below.


The method used to separate the isomers and identify the products are as follows:
1) The excess dimethylmaleic anhydride was separated from the crude mixture by sublimation, after which recrystallisation gave pure benzocantharidin
2) Isobenzocantharidin was obtained from the mother liquor by chromatography on silica gel followed by recrystallisation.
The isomers were differentiated by looking at the NMR data and seeing an upfield shift of the endo methyl protons in the 1H NMR spectrum , and an upfield shift of the endo carbon atoms in the 13C NMR spectra. This assignment of the NMR to isomers was confirmed by an X-ray diffraction of the exo isomer. The data obtained for the literature will be looked at closer and analysed with comparison to the data collected by the different methods mentioned below.
Initially the isomers where drawn in ChemBio3D and the geometry optimised by using MM2 and the total energies can be seen below. They were optimised using the DFT method (mpw1pw91) and were sent to SCAN for geometry optimisation. The optimised geometries were check and the files then edited to be sent for NMR calculations. The literature used chloroform so it was used in the experiment. The IR data for each isomer was also generated by running a b3lyp method.
Below is the MM2 force field total energy data which was used to minimise the energy. It can be seen that the exo product has a lower energy by 1.1 kcal/mol, so is the thermodynamic product and in this case is the more favoured isomer in the literature. This is in agreement with the energy data below.
| Exo | 49.39 |
| Endo | 50.49 |
The 13C NMR data was collected for the exo [11] and endo [12] products and compared against literature for both products as can be seen in the tables below.
| Peak | Coupling carbons | Literature δ/ ppm | Experimental δ/ ppm | Difference lit-exp δ/ ppm |
|---|---|---|---|---|
| 1 | 7,8 | 14.1 | 16.1 | -2.0 |
| 2 | 2,1 | 55.8 | 58.3 | -2.5 |
| 3 | 12,11 | 87.1 | 86.9 | 0.2 |
| 4 | 10,13 | 122.2 | 119.6 | 2.6 |
| 5 | 4,3 | 128.0 | 124.4 | 3.6 |
| 6 | 6,5 | 141.2 | 139.3 | 1.9 |
| 7 | 15,14 | 174.2 | 173.5 | 0.7 |
| Peak | Coupling carbons | Literature δ/ ppm | Experimental δ/ ppm | Difference lit-exp δ/ ppm |
|---|---|---|---|---|
| 1 | 7,8 | 16.8 | 19.5 | -2.7 |
| 2 | 2,1 | 57.9 | 62.3 | -4.4 |
| 3 | 12,11 | 88.3 | 90.7 | -2.4 |
| 4 | 10,13 | 121.2 | 119.2 | 2.0 |
| 5 | 4,3 | 128.7 | 125.4 | 3.3 |
| 6 | 6,5 | 140.5 | 138.1 | 2.4 |
| 7 | 15,14 | 171.6 | 176.0 | -4.4 |

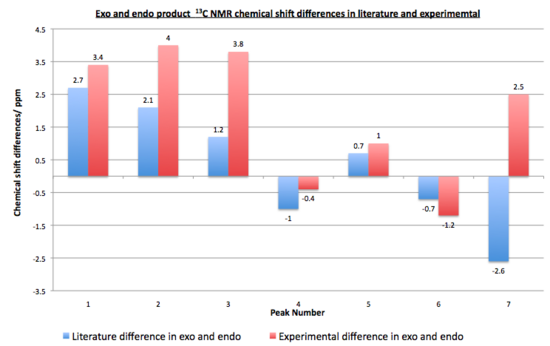
The literature and data collected are similar as can be seen from the differences. All of the values have a difference of less than 5ppm. The graph above shows graphically the differences between each peak for both isomers. The graphs show a small deviation of the literature values to the experimental ones. In most cases the literature is slightly higher than the values obtained, however they are accurate enough to see the difference in the two isomers NMR's. The endo isomer has a larger range of data of 7.7ppm whereas the exo is 6.1ppm. This can also be seen from the graphs below. If a more statistical calculation is looked to analyses the differences then the root mean square deviation can be looked at, it is a good measure of precision. For the exo product it was 2.21 ppm and for endo product 3.22 ppm. These values are again below 5 ppm and indicate that the 13C NMR data collected experimentally matched well to the literature data, so it will be an accurate tool to use when trying to differentiate between the isomers.
Taking a closer look at the individual isomers data both in literature and the ones calculated. Both products give the same number of peaks as expected. In literature the NMR stereochemical assignments were made on the basis of an upfield shift of the endo carbons in the 13C NMR spectra for the endo isomer. This can also be seen when looking at peaks 6 and 7 which are at lower ppm in both peak 6 and 7 in the literature. This maybe due these carbons being less deshielded due to them being on the same face as the bridged oxygen so has less influence from the dimethylmaleic anhydride to deshield it, unlike the exo product which has the same carbons more aligned so will be more deshielded. These correspond to the carbons 5, 6 and 15,14 coupling peaks to be lower for the endo then the exo product. This was the case for peak 6 but not 7 in the computed data. The other peaks assigned for the endo and exo products can also be compared. Peak 1 for C7 and C8 was larger in the endo then exo in literature and this is also the case in the computed data. This is the case for all the peaks where the difference in the literature data between the chemical shifts in isomers is reflected in the experimental data, except the last peak. This can be seen clearer from the bar graph below, when the difference in the endo and exo in literature were compared to the difference in the experimental data. All the peaks except the last one follow the same trend in the differences. The experimental data has larger differences in ppm between the isomers then the literature.
The literature also contained 1H NMR data however this could not be compared, as the computational data is not as reliable for 1H shifts. The three bond coupling can however be predicted reasonable well using a method based on the Karplus equation. The Janocchio tool was used by inputting MDL Molfiles into the program and calculating the 3J HH values. There were only three sets of these and the atom numbers correspond to the ones in figure 10 and 11. They were the ones in the phenyl ring. They can be seen below; the endo coupling constants for two sets are slightly lower. This would have been useful to analyse if the literature had any coupling constants, but this was not the case. This spectroscopic method is not as useful as the 13C NMR data, as the differences in the isomers values are not as distinct. It does however highlight the limitations of other methods and shows how useful others can be.
| Coupling atoms | Exo 3J HH | Endo 3J HH |
|---|---|---|
| H10H11 | 8.2200 | 8.2199 |
| H11H12 | 8.2200 | 8.2200 |
| H12H13 | 8.2200 | 8.2199 |
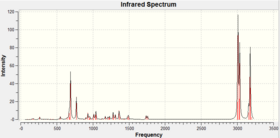
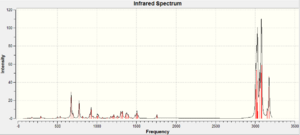
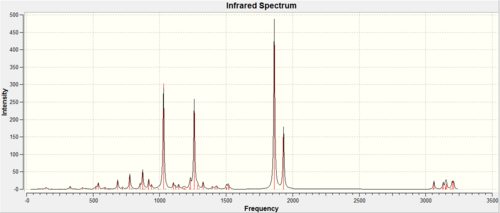
IR data for both the exo [13] and endo [14] product are shown below, frequencies with large intensities were examined. There was not an extensive IR data report in the literature and both isomers were assigned the same peaks : 1850 and 1780 cm-1. To verify the similarity in the IR spectra the data was collected and the comparison can be seen below.
If the two literature peaks are looked at 1850 and 1780 cm-1 for the C=O peak. These were both seen in the IR spectra with the difference in endo and exo being 0.7 cm-1, therefore agreeing with the literature for both isomers to have the same values. However other peaks where not mention in the literature and the main peaks computed are shown below. The value differences between the two isomer is very small ranging from 0.3 to 9.5 being the largest frequency different in the two isomers. So the IR from the experiment confirms that the literature data was correct to assign the two isomers with the same peaks. From an initial glance at the IR it can be seen the peaks are very similar but some intensities are not, as shown in figure 12 and 13.
IR spectroscopy is therefore not the most useful in analysing the prodcuts, it is a preferred method for identifying the functional groups which are the same for both isomers. There are also errors in the calculation due to the calculations being done in the gas phase and the literature being ran in a solvent at room temperature. The errors in predicted wavenumbers are systematically too high for stretch by around 8% and bending and lower frequency modes are normally about right. The accuracy of the IR data cannot be analysed as extensively as all peaks were not found in literature. IR would not be the more obvious technique to use to differentiate the two isomers and the NMR above would be a much better alternative.

| Exo frequency/ cm-1 | Intensity | Endo frequency/ cm-1 | Intensity | Peak assignment |
|---|---|---|---|---|
| 539.3 | 18.2 | 535.3 | 19.6 | Phenyl CH bend |
| 685.0 | 27.1 | 689.1 | 31.1 | Phenyl CH bend |
| 776.0 | 44.2 | 779.1 | 24.7 | CH bend |
| 872.4 | 54.1 | 875.8 | 43.1 | CH bend |
| 1029.1 | 302.6 | 1023.3 | 239.4 | C-O (bridgehead) |
| 1103.6 | 17.1 | 1109.0 | 0.3 | CH3 stretch |
| 1260.0 | 257.1 | 1250.5 | 117.2 | C-O stretch |
| 1327.2 | 19.7 | 1322.1 | 6.4 | CH3 bend |
| 1861.7 | 486.8 | 1862.4 | 424.4 | C=O |
| 1931.6 | 177.9 | 1929.8 | 102.7 | C=O |
| 3062.6 | 21.1 | 3062.2 | 29.0 | CH stretch |
| 3209.0 | 19.9 | 3212.6 | 17.2 | Phenyl CH stretch |
Other Spectroscopic techniques
The methods used above helped to identity the differences between the isomers, where some methods were better than others. In the literature there were other methods used to differentiate between the isomers. Both the Rf and melting point is higher for the exo product. So these would be important tools to also use to differentiate between the products. The endo product is described as being colourless leaflets and the exo colourless crystals, this visual observation is useful to identify the two different isomers. This however a more qualitative analysis and the quantitative spectroscopic method would be more accurate.
| Exo | Endo | |
|---|---|---|
| Rf (silica gel, benzene) | 0.22 | 0.17 |
| Melting point oC | 180-181 | 152.5-153.0 |
Another method which was mention in the literature is X-Ray diffraction. The exo product has also being studied in another report, [15] and is also a very useful technique to distinguish between the two isomers. The exo structure was found to be almost identical to the cantharidon molecule with some distortions due to the added aromatic ring. The internal angle of the saturated six membered ring with the fused aromatic ring increased as the C-C bond shortened. The product was crystallised from diethyl ether. The goal of the X-ray diffraction was to show the endo stereochemistry of the methyl C(14) and C(15) labelled in figure 10. The report was also able to analyse the intermolecular contacts and suggest there are contacts between O(18)-C(11),O(18)- C(12) and O(16)-C(11) of about 3Å as labelled in figure 10.
The X-Ray analysis is also useful as is it able to give information about the π electron density increasing on the exo side of the product. It is suggested that the uneven distribution of the π-electron density could play a role in the more preferred reactivity of the norbornene at the exo face. Therefore the non-planar π-bond could affect the reactivity of the carbocyclic π-system. Therefore not only is the X-ray data useful for analysing the isomers but also looking at the reactivity.
Isomer variation
Above is the discussion of how to identify each isomer with different techniques. It will now be looked at as to how the different conditions give rise to each isomer.[10] The selectivity has come from the reagents and conditions used, the isobenzofuran has good Diels-Alder reactivity due to the favourable changes in aromatic stabilization in the cycloaddition. This was instead of furan, which has minimal effect on the steric factors of the transition state in cycloaddition. The 95:5 ratio in favour of the desired exo isomer, was also brought about by the high-pressure used in the experiment.
Looking closer at the reagents the ratio can be alter, however most reactions still give the exo product as being the dominant isomer. In the reaction being analysed above the excess dimethylmaleic anhydride was separated from the crude mixture by sublimation and recrystallisation to give benzocantharidin. The isobenzocantharidin was obtained by chromatography on silica gel followed by recrystallisation. The exo isomer as the major product formation is explained due to the relative thermodynamic stability. This was also found with the MM2 energies above. It is also due to the reversible cycloaddition reaction. This was also the case when the Diels Alder reaction was ran in toluene. It was refluxed in toluene for 4 hours and gave a 90:10 and when left for 15 hours a 93:7 exo/endo product ratio.
The major isomer formation can also be explained due to the reasoning below. When the endo isomer was refluxed for 15 hours in toluene there was a exo/endo ratio of 2:3. However when it was refluxed without the dimethylmaleic anhydride, the endo isomer lead to partial destruction of the cycloadduct. This was because the endo product polymerised by a retro-Diels-Alder reaction. Therefore it can be seen that the preferred isomer is the exo one as there is intermolecular process that can compete with polymerisation of product when there is excess dimethylmaleic anhydride present, unlike the endo product above. Again this was seen when the exo isomer was refluxed in toluene for 7 hours and was stable. However under extreme high temperatures the product did undergo the retro-Diels-Alder reaction, but otherwise was stable.
Conclusion
The computational methods used above for the mini-project showed they were good ways to identify different products in a reaction and helped identify the isomers in the literature. The 13C NMR data was the most useful in analysing the isomers and agreed with the literature well. There were however some limitation in not being able to generate 1H NMR due to the inaccuracy. The IR data also collected matched the literature but was not as useful to differentiate between the two isomers. Therefore with the additional use of X-ray diffraction data and 1H NMR the isomers identification could have been further improved. Overall the computational methods have provided enough data to be able to see the uses in trying to analyse differences in isomers.
References
- ↑ K. Alder and G. Stein , Liebigs Ann.,1933,504 ,216
- ↑ J.Furukawa, Y.Kobuke and T.Fueno, J. Am. Chem. Soc.,1970, 92(22) ,6548–6553
- ↑ A. G. Shultz, L. Flood and J. P. Springer, J. Org. Chemistry, 1986, 51, 838 DOI:10.1021/jo00356a016
- ↑ S. Leleu, C. Papamicael, F. Marsais, G. Dupas, V.Levacher, Vincent. Tetrahedron: Asymmetry, 2004, 15, 3919-3928
- ↑ S. W. Elmore and L. Paquette, Tetrahedron Letters, 1991, 319; DOI:10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0 10.1016/S0040-4039(00)92617-0
- ↑ Frank S. Fawcett, Chem. Rev., 1950, 47 (2), 219–227
- ↑ SPECTRa Chemical Repository (IR dicholorcarbene) :DOI:/10042/to-6409
- ↑ SPECTRa Chemical Repository (IR monocholorocarbene) :DOI:/10042/to-6400
- ↑ B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2 , 1992 , 447. DOI:10.1039/P29920000447
- ↑ 10.0 10.1 J.P.McCormick and T.Shinmyozu, J. Org. Chem., 1982, 47 (21), 4011–4012
Cite error: Invalid
<ref>tag; name "10th" defined multiple times with different content - ↑ SPECTRa Chemical Repository (NMR exo) :DOI:/10042/to-6414
- ↑ SPECTRa Chemical Repository (NMR endo) :DOI:/10042/to-6415
- ↑ SPECTRa Chemical Repository (IR exo) :DOI:/10042/to-6439
- ↑ SPECTRa Chemical Repository (IR endo) :DOI:/10042/to-6441
- ↑ E.O.Schlemper, J.P.McCormick and T.Shinmyozu, Acta Cryst., 1982, 2981-2983.
