Rep:Mod:1SC
Introduction
This lab assumes that you are already familiar with the first and second year Gaussian [1] labs. Please revise these before beginning work here. A Lab Toolbox is provided and you should consult this before beginning the lab.
In this lab, if you have already conducted the synthetic experiment, are encouraged to cite your findings and compare results from computational simulations with your results. This is what happens in projects!
You should use Gaussian 09/ Gaussview [1] to conduct calculations. Some of your calculations may take several hours to run, even on the HPC! As such, we advise that you use your time wisely. For example, you could run your calculations overnight (but make sure that you know that they won't fail within one second of running!) and write up your wiki and conduct research and run smaller calculations during the day. It is completely up to you where you work or when you run your simulations.
A Lab Toolbox is provided and you should consult this as you progress through this lab. You will need to conduct research and consult experimental results/ other published computational simulations at various points in order to verify the validity of your results - this is what happens in computational research!
Intended Learning Outcomes
This lab follows on from the 1S Synthesis Lab of the Jacobson and Shi catalysts which you conducted/will conduct this year. By the end of this lab, you should be able to:
Aims
1) Understand and appreciate how to select a basis set for computational calculations.
2) Conduct complex computational calculations
3) Analyse data effectively and efficiently from the outputs of computational simulations
Objectives
1) Determine the stability of isomers. This can help you understand which one may be formed in excess in a reaction.
2) Calculate the Optical Rotations of your epoxides. In the lab, this can be used to determine which enantiomer you have an excess of. Computational Simulations also allow you to calculate such properties.
3) Determine the enantiomeric excess of your products via transition state calculations.
Part 1: Basis sets
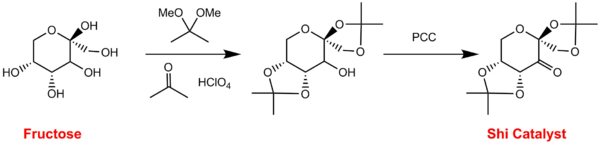
Basis sets are used in computational chemistry (those programs which use Gaussian Type Orbtials) as a method to approximate the atomic orbitals in a chemical system. Fructose is a precursor to the Shi catalyst (Fig. 1) and you should use fructose as a base molecule to determine which basis set you will use to run any further calculations. You will need to use the B3LYP functional at all times during this work.
Basis sets for selection are: 3-21G, 6-31++G(d,p) and 6-311++G(d,p)
Determine which of these basis sets will provide a good degree of accuracy to your calculations. Discuss the difference between the basis sets and justify your selection, making a critical evalutation. You may use the desktop computers (via the Software Hub)or the HPC.
Part 2: Epoxides and their reactions
Four different alkenes are investigated with in the 1S lab: styrene, trans-β-methylstyrene, trans-Stilbene and 1,2-Dihydronaphthalene.
Upon oxidation, these alkenes all form the corresponding epoxide, where the oxygen can either be distinguished as being face up or down for some alkenes. This leads to a pair of enantiomers for each of the epoxides. You can run these calculations either on the College Desktop computers or the HPC. Determine a method to distinguish between the two enantiomers of the epoxide produced; run a calculation to verify they are enantiomers and discuss your results. Are the results reliable? What other methods could you use?
Trans-β-methylstyrene, forms trans-β-methylstyrene oxide after oxidation with the Jacobson and Shi catalysts. Reaction of an epoxide with a nuclophile, NH2Ph, yields new products. In fact, research was conducted by Kureshy et al who used Jacobson like catalysts to do the above. [2]
Determine the different products which could be formed and discuss their relative energies. Which is the most stable; can the kinetic and thermodynamic product be determined from this information?
Whilst your calculations are running for this part of the lab, we recommend that you continue to Part 3: Enantiomeric Excess (below) of the lab. Calculations on the HPC may take a while to run!
Part 3: Enantiomeric Excess
Computational methods can also be used to calculate the enantiomeric excess of epoxides produced by either the Jacobson or Shi catalyst from a reaction. Determination depends on being able to distinguish between the various transition states.
For this part of the lab, you will need to click here for the script. Do not complete any sections other than those under "Using the (calculated) properties of transition state for the reaction" and do not complete the sections thereafter beginning "Investigating the non-covalent interactions in the active-site of the reaction transition state"
References
- ↑ 1.0 1.1 M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. J. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Wallingford CT, 2013.
- ↑ R. Kureshy, S. Singh, N. H. Khan, H. R. Abdi, S. Agrawal, R. V. Jasra; Tetrahedron Asymmetry; 17; 11; 2006; DOI: https://doi.org/10.1016/j.tetasy.2006.05.029
