Rep:Mod:1CJKIM
Part 1: Conformational Analysis using Molecular Mechanics
Hydrogenation of Cyclopentadiene Dimer
By using computational analogy we are able to predict the energies of different structural isomers and determine where the product is kinetic or thermodynamic.
| Product label | Structure | Energy (kcal/mol) | Stretching Energy (kcal/mol) | Bending Term (kcal/mol) | Torsional Term (kcal/mol) | van der Waals Energy (kcal/mol) | Electronic Energy (kcal/mol) |
|---|---|---|---|---|---|---|---|
| 1 | exo-dimer 1 | 55.3765 | 3.53353 | 30.77219 | -2.70658 | 12.79706 | 13.01035 |
| 2 | endo-dimer 2 | 58.1964 | 3.46129 | 33.29236 | -2.08089 | 12.28731 | 14.18083 |
| 3 | derviative 3 | 50.4476 | 3.35256 | 29.01327 | -0.22012 | 13.48985 | 5.11295 |
| 4 | derviative 4 | 41.2719 | 2.83315 | 24.67437 | -0.36224 | 10.62719 | 5.14678 |
Looking at the energies above, the molecule 1 has lower in energy than 2. The two molecules have similar stretching, bending and van der Waal's energy. The highest difference in energy lies in the torsional strain, where the energy of the endo-dimer is greater than the exo- form. This shows that the endo-dimer is the kinetic product while the exo-dimer is the exothermic product.
The difference in torsional energy can be explained in terms of difference in structure and the overlap of orbitals. The two rings are much closer for the endo-dimer and hence the overlap of secondary orbitals is much more favoured.
However, for the reaction where 1 and 2 are products it is known that 1 is more favoured (95% of product is the kinetic product [1]), despite the kinetic product being higher in energy.This is because the dimerisation reaction is irreversible and a reversible reaction would favour the more energetically stable thermodynamic product.
For the hydrogenation of the dimer, the energy of derivative 4 is lower than derivative 3 hence the reaction would lead to formation of more thermodynamically stable derivative 4. Derivative 4 is the product from the hydrogenation of the double bond in a norbornene ring and derivative 3 is the product from the hydrogenation of double bond in cyclopentene. The activation energies of the hydrogenation of the double bond for norbornene ring is 7.8945 kj/mol and for cyclopentene is 12.2068 kJ/mol [2]. So the energy for breaking of the double bond to produce 4 is much lower, which supports the thesis that 4 will form faster than 3.
The molecule of Taxol 9 was modelled and the isomers of the lowest energy was found using the MMFF94 force-field minimization. The results showed that there are four different isomers present and the same method was applied to Taxol 10:
| Product label | Structure | Energy (kcal/mol) | Comment |
|---|---|---|---|
| 9a | 9a | 63.2840 | Cyclohexane in chair formation with hydrogen atoms pointing downwards |
| 9b | 9b | 77.9460 | Cyclohexane in boat formation with hydrogen atoms pointing upwards |
| 9c | 9c | 67.7616 | Cyclohexane in a twist-boat formation with hydrogen atoms pointing downwards |
| 9d | 9d | 70.5517 | Cyclohexane in chair formation with hydrogen atoms pointing upwards |
| 10a | 10a | 66.3382 | Cyclohexane in boat formation with hydrogen atoms pointing upwards |
| 10b | 10b | 60.5749 | Cyclohexane in chair formation with hydrogen atoms pointing upwards |
| 10c | 10c | 73.3024 | Cyclohexane in chair formation with hydrogen atoms pointing downwards |
| 10d | 10d | 76.4797 | Cyclohexane in twist/boat formation with hydrogen atoms pointing downwards |
The most energetically stable isomer was chosen for each Taxol molecule and their structure and energies were studied in more detail:
| 9a | 10b | ||||||
|---|---|---|---|---|---|---|---|
|
|
| Product label | Energy (kcal/mol) | Stretching Energy (kcal/mol) | Bending Term (kcal/mol) | Torsional Term (kcal/mol) | van der Waals Energy (kcal/mol) | Electronic Energy (kcal/mol) |
|---|---|---|---|---|---|---|
| 9 | 63.2840 | 7.55909 | 21.58844 | -0.52025 | 33.50514 | 0.28626 |
| 10 | 60.5749 | 7.61757 | 18.89088 | 0.32432 | 33.12722 | -0.04929 |
The results showed that the isomer with the lowest energy is 10b with 60.5749 kcal/mol. This is therefore the most stable conformation. The molecule has its cyclohexane ring in a chair conformation and this allows C-H bonds to be in staggered position which reduces torsional strain. The other conformations such as the boat and twist-boat are less effective in relieving torsional strain due to having the C-H bonds in an eclipsed position.
Two structural isomers of an intermediate related to the synthese of Taxol were minimised using the MMFF94 option and its energy was calculated. The structure and energy of the two intermediates labelled 17 and 18 are shown below:
| Molecule | Structure | Energy (kcal/mol) |
|---|---|---|
| 17 | 17 | 104.995 |
| 18 | 18 | 104.668 |
The molecule of Taxol derivative 18 was chosen since it is the isomer of lower energy than derivative 17. Therefore it can be regarded as the more thermodynamically stable compound. The NMR spectra of this molecule was predicted using the HPC system:




The results from the computational analysis were then compared with values from literature[3] :
| Carbon-13 NMR values from literature (ppm) | Carbon-13 NMR values in chair conformation (ppm) | Carbon-13 NMR values in boat conformation (ppm) |
|---|---|---|
| 211.49 | 216.23 | 211.83 |
| 148.72 | 142.23 | 148.28 |
| 120.90 | 123.23 | 117.93 |
| 74.61 | 92.12 | 83.45 |
| 60.53 | 65.82 | 67.31 |
| 51.30 | 53.42 | 55.20 |
| 50.94 | 52.19 | 56.11 |
| 45.53 | 51.23 | 55.13 |
| 43.28 | 47.58 | 49.05 |
| 40.82 | 42.63 | 46.56 |
| 38.73 | 40.14 | 44.15 |
| 35.47 | 38.51 | 40.67 |
| 30.84 | 33.70 | 31.25 |
| 30.00 | 29.53 | 28.85 |
| 25.56 | 25.85 | 25.79 |
| 25.35 | 25.26 | 25.19 |
| 22.21 | 24.56 | 22.83 |
| 21.39 | 22.01 | 20.42 |
| 19.83 | 21.53 | 20.13 |
The carbon-13 NMR was chosen to be analyzed since it is much easier to observe the difference between calculated and literature values. The carbons with higher ppm values (from 74 ppm to 91 ppm) tend to deviate more from the values in literature. These carbon atoms are identified as the ones that are connected to the two sulfur atoms and hence the values are changed a lot more than the others. Overall, neither of the NMR spectrum data is an exact match to the values obtained in literature therefore it is difficult to determine whether the molecule has the cyclohexane ring in boat formation or chair formation.
Thermochemistry


The values of the Gibbs free energy were -1651.464675 (in Hartrees/particle) and -1651.469893 for chair and boat conformations respectively. This shows that the chair conformation has a slightly lower in energy than the boat. By just looking at the energies of the molecule, a prediction can be made that the chair conformation is more thermodynamically stable.
Part 2: Analysis of the properties of the synthesised alkene epoxides
Crystal Structure of the Shi and Jacobsen Catalyst
The crystal structures of the Shi and Jacobsen Catalyst was found using the Cambridge Crystal Database via the Conquest search engine.
Shi |
The C-O bond lengths of the O-C-O anomeric centres were obtained. The C-O bond lengths of on centre was 1.42A and 1.39A while the other had 1.38A and 1.415A. The difference in bond lengths can be explained by anomeric effect. The longer the bond length, the weaker the bond becomes. The shorter C-O bond is in an ideal position where the electron density can be donated from the oxygen lone-pairs of the ring. This bond is strengthened while the other C-O bond is unable to receive electron density.
Jacobsen |
NMR of Styrene oxide and trans-stilbene oxide
The two types of alkene oxides chosen for the experiment were styrene oxide and trans-stillbene oxide. Their NMR spectrum was predicted using the HPC system and uploaded on Dspace [4] [5] [6] [7] .
The results were compared with the literature values and were found be in good agreement of each other. However, it was difficult to compare the data since the literature did not indicate the exact proton environment for the range of chemical shifts.
| Chosen epoxide | Structure | Energy (kcal/mol) | |||
|---|---|---|---|---|---|
| styrene oxide |
|
21.6362 | |||
| trans-stilbene oxide |
|
39.5445 |




Calculation of the chiroptical properties of the product
The optical rotation of R- and S-styrene oxide and trans-stilbene oxide were calculated and submitted on Dspace [8] [9] [10].
The wavelength of light used for the calculation was 589 nm for styrene oxides. The results gave values of -30.49 degrees and 30.27 degrees for R-styrene oxide and S-styrene oxide respectively. These were in good agreement with the values from literature which were -33.3 degrees [11]and 32.1 degrees [12] respectively for R- and S-styrene oxide.
Thermochemistry
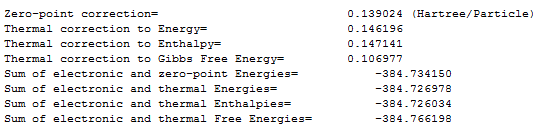

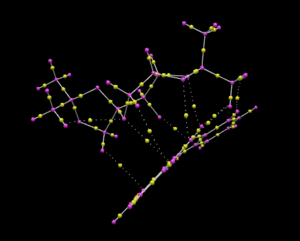
Investigating the non-covalent interactions in the active-site of the reaction transition state

The non-covalent interactions include hydrogen bonding, electrostatic interactions and dispersion interactions between atoms. The NCI analysis of the (R,R)-stilbene oxide and shi catalyst transition state was run using Gaussview. From the diagram above, the green surface shows the non-covalent interactions between the two molecules. The colour green indicates that the interactions are weak but indicate that the are attractive forces which is evidence to show a successful reaction can take place.
Electronic Topology (QTAIM)
The further investigation was done to look the bond formation in the TS. An electronic topology study was made using the Avogadro2 programme. From the diagram, a bond critical point (BCP) is shown in yellow spheres and this is where the two atoms of bond is at lowest density. The evidence of hydrogen bonding is seen (also identified in the NCI interation) due to the presence of BCPs between the oxygen atoms of Shi catalyst and the hydrogen atom attached to carbon of (R,R)-stilbene molecule. Also,a BCP was observed to be present between one of the oxygen atoms on the Shi catalyst and the olefin carbon atom. This is an evidence to show that the C-O bond made from oxygen of Shi and carbon atom of R,R-stilbene is not a concerted formation.
Suggested Epoxide
A further analysis can be done on methyloxirane, which is a small epoxide which has an optical rotation btween -300° and 300°. The optical rotation does change with presence of solvent, but in the absence the value at wavelength of 589 nm is 13.2°[13].
.
References
- ↑ J. D. Rule and J. S. Moore, Macromolecules, 2002, 35, 7878–7882DOI:10.1021/ma0209489
- ↑ G. Liu, Z. Mi, L. Wang, and X. Zhang, Ind. Eng. Chem. Res., 2005, 44, 3846–3851.DOI:10.1021/ie0487437
- ↑ L. A. Paquette, N. A. Pegg, D. Toops, G. D. Maynard, and R. D. Rogers, J. Am. Chem. Soc., 1990, 112, 277–283.DOI:10.1021/ja00157a043
- ↑ J. Kim, R-styrene oxide, 2014 DOI:10042/27958
- ↑ J. Kim, S-styrene oxide, 2014 DOI:10042/27959
- ↑ J. Kim,(S,S)-trans-stillbene oxide, 2014 DOI:10042/27960
- ↑ J. Kim,(R,R)-trans-stillbene oxide, 2014 {{DOI|10042/27961
- ↑ J.Kim,R-styrene oxide OR,2014.DOI:10042/27964
- ↑ J.Kim,S-styrene oxide OR,2014.DOI:10042/27965
- ↑ J.Kim,trans-stilbene oxide OR,2014.DOI:10042/27966
- ↑ F. R. Jensen and R. C. Kiskis, J. Am. Chem. Soc., 1975, 97, 5825–5831.DOI:10.1021/ja00853a029
- ↑ H. Lin, Y. Liu, and Z.-L. Wu, Tetrahedron: Asymmetry, 2011, 22, 134–137.DOI:10.1016/j.tetasy.2010.12.022
- ↑ M. Inoue, K. Chano, O. Itoh, S. Osamu, T. Sugita and K. Ichikawa, Bulletin of the Chemical Society of Japan, 1980, 53(2), 458-463
