Rep:Mod:1990DZC
Computational Lab, Physical
In this module, we characterised transition structures in larger molecules for the Cope Rearrangement and the Diels-Alder reaction.
Shapes of optimised starting materials, products and transition structures were calculated as well as reaction pathways and barrier heights.
The Cope Rearrangement tutorial
The Cope Rearrangement of 1,5-hexadiene was studied in this module. This [3,3]-sigmatropic rearrangement is an example of pericyclic reaction which has a cyclic-geometric transition state and its reaction progresses are in a concerted fashion.

To determine the mechanism of the Cope Rearrangement, different comformations (6 gauche and 4 anti) of 1,5-hexadiene were calculated and compared.
Optimising the Reactants and Products
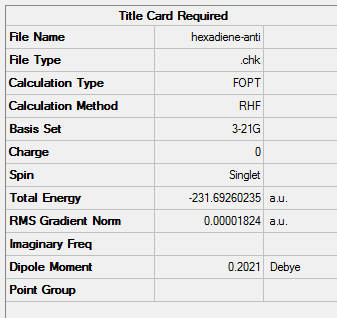
(a) Optimisation of 1,5-hexadiene with an "anti" central linkage
A 1,5-hexadiene molecule was drawn by combing a ethyl fragment and two vinyl fragments together and its dihedral angles were modified to give an anti-central linkage. This 1,5-hexadiene molecule was then optimiesd with HF/3-21G.
1. Optimising 1,5-hexadiene (anti) using HF/3-21G
test molecule |
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000056 0.000450 YES
RMS Force 0.000010 0.000300 YES
Maximum Displacement 0.001357 0.001800 YES
RMS Displacement 0.000459 0.001200 YES
Predicted change in Energy=-2.090841D-08
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
5. Key information
| Linkage | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
| Anti | Optimisation to a minimum | HF | 3-21G | 250 MB | -231.69260235 a.u. | C2 |
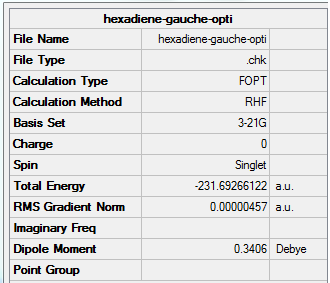
(b) Optimisation of 1,5-hexadiene with an "gauche" central linkage
This molecule was drawn by changing the dihedral angles of the molecule in (a).
1. Optimising 1,5-hexadiene (gauche) using HF/3-21G
test molecule |
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000014 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000463 0.001800 YES
RMS Displacement 0.000153 0.001200 YES
Predicted change in Energy=-3.377162D-09
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
5. Key information
| Linkage | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
| Gauche | Optimisation to a minimum | HF | 3-21G | 250 MB | -231.69266122 a.u. | C1 |
6. Comparison with (a)
| Energy (a) | Energy (b) | Energy difference (b)-(a) |
|---|---|---|
| -231.69260235 a.u. | -231.69266122 a.u. | -0.00005887 a.u. |
Higher energy was expected than (a) as two vinyl groups are closer in space leading to larger steric repulsion. However, the final energy of the optimised gauche structure is lower and the energy difference is equal to 0.00005887 a.u.(or 0.0369414 kcal/mol).
This is because the gauche conformation has a better sigma-sigma* interaction between bonding C-C orbital and antiperiplanar antibonding C-H orbital compared to the anti conformation. Moreover, goauche3 conformation is the most stable because good C-H-pi interaction between two vinyl groups. The H on one vinyl group is delta+ due to its sp2 geometry, so it has good interaction with electron rich pi orbital on the other vinyl group.
(c) Optimisation of lowest energy conformation of 1,5-hexadiene
Results exactly as (b).
(d) Identification of optimised structures
| Optimised stucture | Conformer identified from Appendix 1 |
|---|---|
| (a) | Anti1 |
| (b) | Gauche3 |
| (c) | Gauche3 |
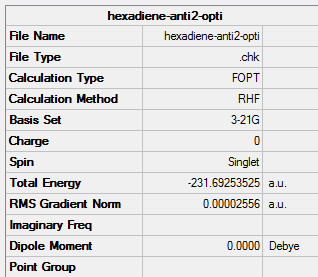
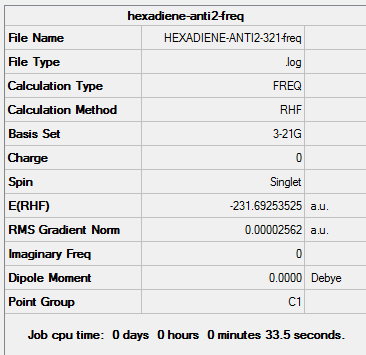
(e) Optimisation of anti2 conformer using HF/3-21G
1. Optimising 1,5-hexadiene (anti2) using HF/3-21G
test molecule |
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000039 0.000450 YES
RMS Force 0.000014 0.000300 YES
Maximum Displacement 0.000564 0.001800 YES
RMS Displacement 0.000177 0.001200 YES
Predicted change in Energy=-5.156886D-08
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
5. Key information
| Conformer | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
| Anti2 | Optimisation to a minimum | HF | 3-21G | Default | -231.69253525 a.u. | Ci |
6. Comparison with Appendix 1
| Energy (optimised) | Energy (Appendix 1) |
|---|---|
| -231.69253525 a.u. | -231.69254 a.u. |
The energy for the optimised structure is very similar to the energy of anti2 comformation in Appendix 1, confirming the structures are the same.
(f) Reoptimisation of anti2 conformer using B3LYP/6-31G(d)
A better basis set i.e. B3LYP/6-31G(d) was used to reoptimise the anti2 conformer in order to get higher accuracy.
1. Optimising 1,5-hexadiene (anti2) using B3LYP/6-31G(d)
test molecule |
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000016 0.000450 YES
RMS Force 0.000007 0.000300 YES
Maximum Displacement 0.000260 0.001800 YES
RMS Displacement 0.000089 0.001200 YES
Predicted change in Energy=-1.717103D-08
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
5. Key information
| Conformer | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
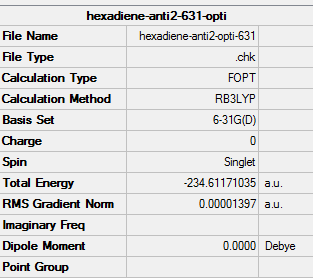
| Anti2 | Optimisation to a minimum | B3LYP | 6-31G(d) | Default | -234.61171035 a.u. | Ci |
7. Comparison with (e)
| Energy (HF/3-21G) | Energy (B3LYP/6-31G(d)) | Energy difference |
|---|---|---|
| -231.69253525 a.u. | -234.61171035 a.u. | 2.91916830 a.u. |
The energy of B3LYP/6-31G(d) optimised structure is much lower than that of HF/3-21G optimised structure, and the energy difference is equal to 2.91916830 a.u.(or 1831.80575 kcal/mol). However, there are no visible differences between the two structures by simply looking at their structures on GaussView as the following is shown.
| HF/3-21G | B3LYP/6-31G(d) | |||||||
|---|---|---|---|---|---|---|---|---|
| Structure |
|
|
To find the out the change in geometry responsible for the large energy difference, the geometric data between the two structures were compared and showed by the table below.
| Geometric parameter | HF/3-21G | B3LYP/6-31G(d) |
|---|---|---|
| C1=C2 (or C5=C6) bond length | 1.31615 Å | 1.33352 Å |
| C2-C3 (or C4-C5) bond length | 1.50880 Å | 1.50421 Å |
| C3-C4 bond length | 1.55284 Å | 1.54808 Å |
| C1=C2-C3-C4 (or C3-C4-C5=C6) dihedral angle | +(or-)114.68828o | +(or-)118.58831o |
From the data above, geometry change was very small and negligible. The largest difference was in dihedral angles and this may cause large energy difference as the double bonds have a better alignment with the neighbouring C-C/C-H bonds, resulting in strong σ-π conjugations and thus have a large stablisation in energy for the B3LYP/6-31G(d) optimised structure.
(g) Frequency analysis of optimised anti2 structure
The frequency analysis is the second derivative of the potential energy surface of a reaction. The previous optimisation was done properly only if all the vibrational frequencies are real and positive.
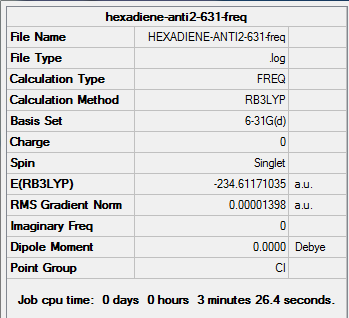
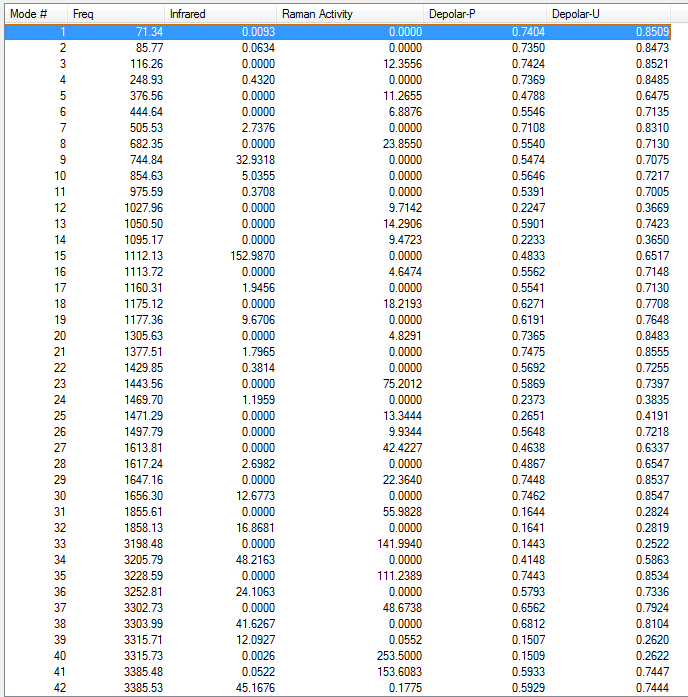
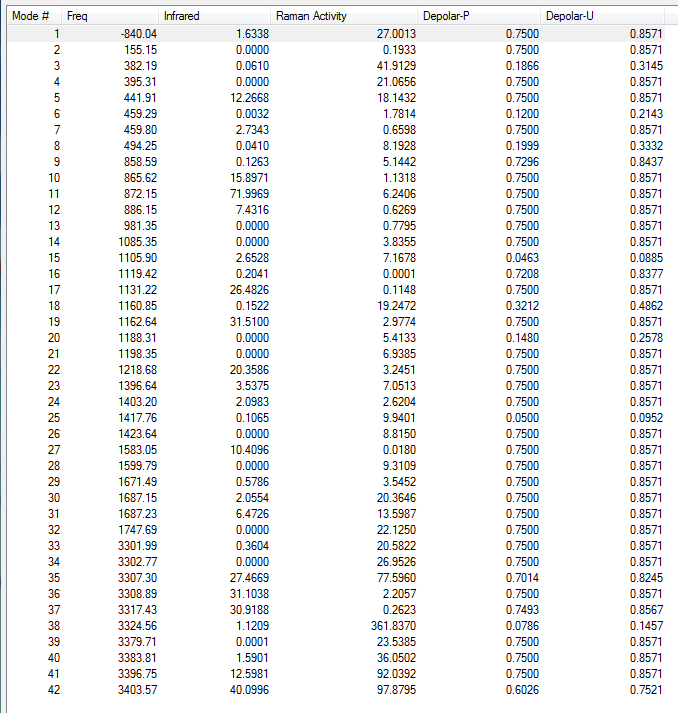
Frequency analysis of B3LYP/6-31G(d) optimised anti2 structure
The energy is the same as that obtained in optimisation, which means the structure is correct (i.e. the same as the optimised structure).
2. Real output
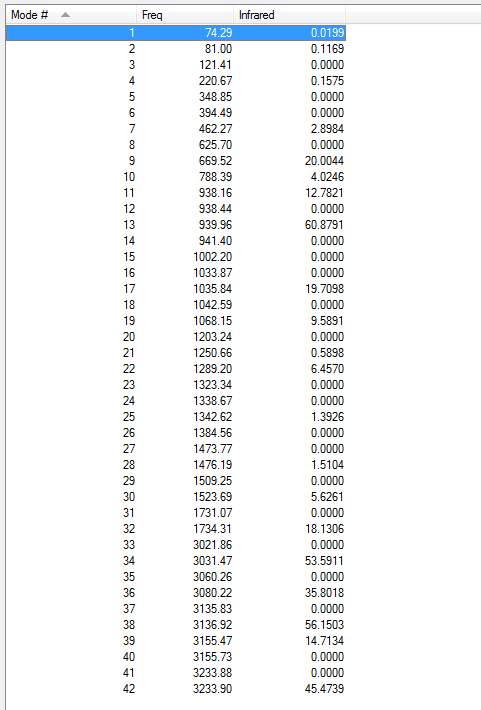
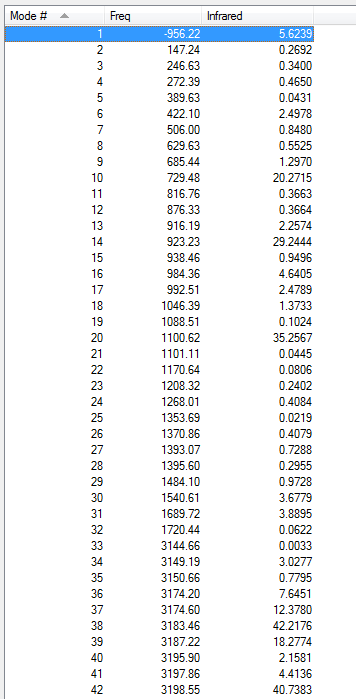
Low frequencies --- -9.4878 -0.0002 0.0005 0.0008 3.7496 13.0251 Low frequencies --- 74.2865 80.9989 121.4178
All vibrational frequencies are real and positive, indicating the molecule is fully optimised to a minimum.
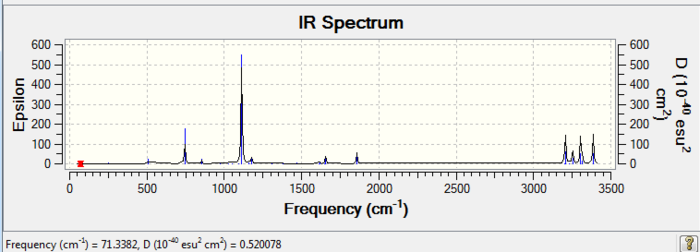
From the vibrational frequencie table and the IR spectrum above, many vibrations have 0 IR absorption intensity therefore are not shown on the spectrum. This is due to hexadiene anti2 conformation is under Ci symmetry hence it is very symmetric. Some symmetric stretches may cancel each other out and therefore IR inactive.
5. Thermochemistry
Sum of electronic and zero-point Energies= -234.469204 Sum of electronic and thermal Energies= -234.461857 Sum of electronic and thermal Enthalpies= -234.460913 Sum of electronic and thermal Free Energies= -234.500777
6. Key information
| Conformer | Job type | Method | Basis set | Memory limit | Energy |
|---|---|---|---|---|---|
| Anti2 | Frequency | B3LYP | 6-31G(d) | Default | -234.61171035 a.u. |
Frequency analysis of HF/3-21G optimised anti2 structure
The energy is the same as that obtained in optimisation, which means the structure is correct (i.e. the same as the optimised structure).
2. Real output
Low frequencies --- -2.2094 -1.6189 -0.0006 -0.0003 -0.0001 6.2740 Low frequencies --- 71.3382 85.7693 116.2625
The low frequencies are within ±15 cm-1.
All vibrational frequencies are real and positive, indicating the molecule is fully optimised.
From the vibrational frequencie table and the IR spectrum above, many vibrations have 0 IR absorption intensity therefore are not shown on the spectrum. This is due to hexadiene anti2 conformation is under Ci symmetry hence it is very symmetric. Some symmetric stretches may cancel each other out and therefore IR inactive.
5. Thermochemistry
Sum of electronic and zero-point Energies= -231.539540 Sum of electronic and thermal Energies= -231.532567 Sum of electronic and thermal Enthalpies= -231.531622 Sum of electronic and thermal Free Energies= -231.570913
6. Key information
| Conformer | Job type | Method | Basis set | Memory limit | Energy |
|---|---|---|---|---|---|
| Anti2 | Frequency | HF | 3-21G | Default | -231.69253525 a.u. |
Optimising the "Chair" and "Boat" Transition Structures
(a) Optimisation of allyl fragment
An allyl fragment was drawn and optimised for further use.
1. Optimising allyl fragment using HF/3-21G
 |
|---|
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000157 0.000450 YES
RMS Force 0.000036 0.000300 YES
Maximum Displacement 0.000636 0.001800 YES
RMS Displacement 0.000277 0.001200 YES
Predicted change in Energy=-7.608588D-08
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
5. Key information
| Fragment | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
| Allyl | Optimisation to a minimum | HF | 3-21G | Default | -115.82304004 a.u. | C2v |
(b) Optimisation of chair transition state by computing force constants
Two optimised allyl fragments were combined and modified to have a conformation very close to a chair transition state.
1. Optimising chair transition state using Berny method with force constants calculation
 |
 |
|---|
The optimised structure looks very similar to the one in Appendix 2 on the right.
2. General information
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000031 0.000450 YES
RMS Force 0.000006 0.000300 YES
Maximum Displacement 0.000600 0.001800 YES
RMS Displacement 0.000150 0.001200 YES
Predicted change in Energy=-2.948570D-08
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
4. Symmetry information
The point group of the optimised structure is C2h, confirming the structure is correct.
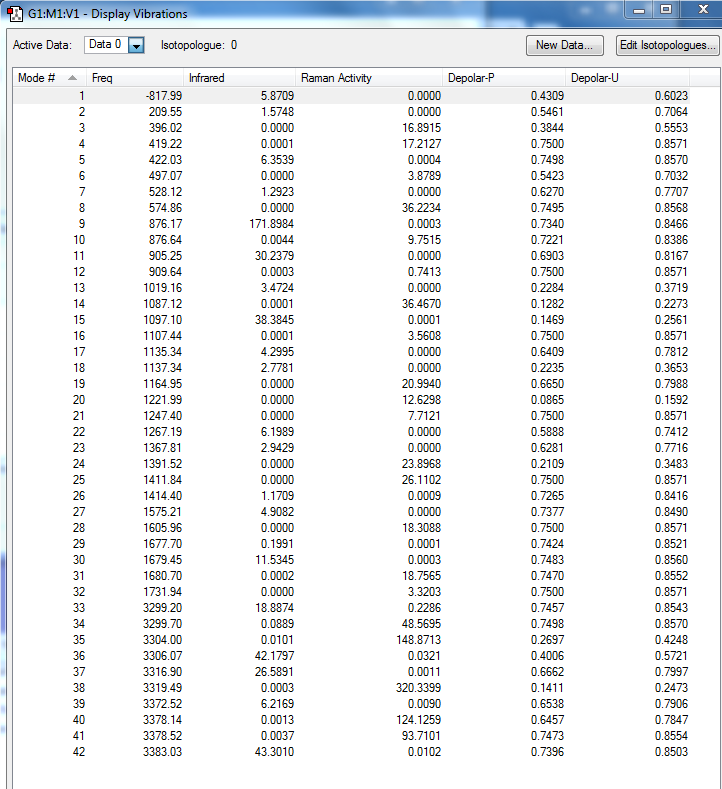
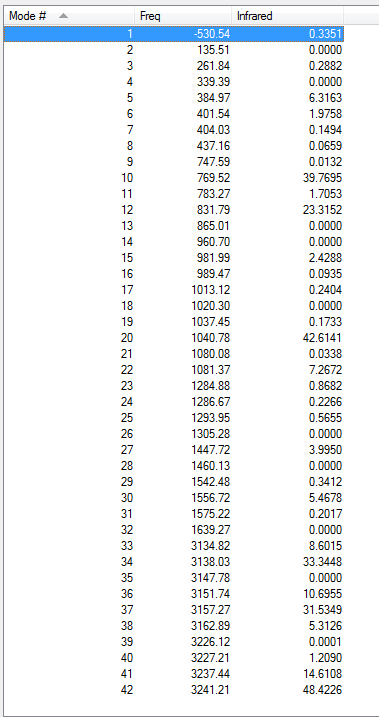
From the table above, only one imaginary frequency that has a magnitude of 817.99 cm-1. It's vibration animation shows there are 2 carbon atoms coming closer at the same time indicating a concerted bond formation and there are 2 carbon atoms leaving far away at the same time indicating a synchronous bond breaking.
6. Thermochemistry
Sum of electronic and zero-point Energies= -231.466700 Sum of electronic and thermal Energies= -231.461340 Sum of electronic and thermal Enthalpies= -231.460396 Sum of electronic and thermal Free Energies= -231.495205
7. Key information
| Transition state type | Job type | Additional keywords | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|---|
| Chair | Optimisation to a TS (Berny), calculate the force constants once | Opt=NoEigen | HF | 3-21G | Default | -231.61932245 a.u. | C2h |
(c) Optimisation of chair transition state using frozen coordinate method
1. Optimising chair transition state with frozen coordinates

The bond distance between the terminal C atoms of the allyl fragments is fixed to 2.2 Å.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000011 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000778 0.001800 YES
RMS Displacement 0.000204 0.001200 YES
Predicted change in Energy=-5.318408D-08
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
The point group of the optimised structure is C2h, confirming the structure is correct.
5. Key information
| Transition state type | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
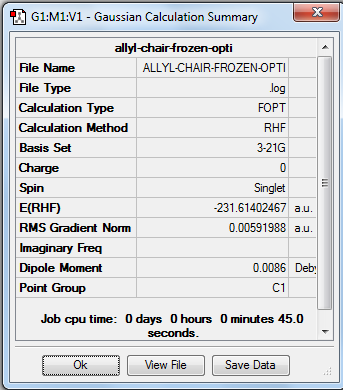
| Chair | Optimisation to a minimum | HF | 3-21G | Default | -231.61402467 a.u. | C2 |
(d) Reoptimisation of chair transition state with unfrozen coordinates
1. Optimising chair transition state using Berny method without force constants calculation
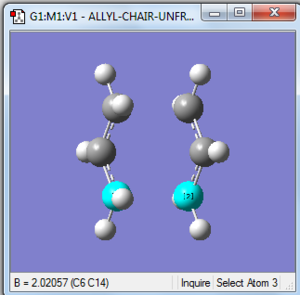
As the GaussView image is shown on the right, this optimised structure looks almost the same as the one optimised in (b).
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000032 0.000450 YES
RMS Force 0.000009 0.000300 YES
Maximum Displacement 0.001666 0.001800 YES
RMS Displacement 0.000315 0.001200 YES
Predicted change in Energy=-3.021453D-07
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
The point group of the optimised structure is C2h, confirming the structure is correct.
6. Thermochemistry
Sum of electronic and zero-point Energies= -231.466705 Sum of electronic and thermal Energies= -231.461344 Sum of electronic and thermal Enthalpies= -231.460400 Sum of electronic and thermal Free Energies= -231.495211
7. Key information
| Transition state type | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
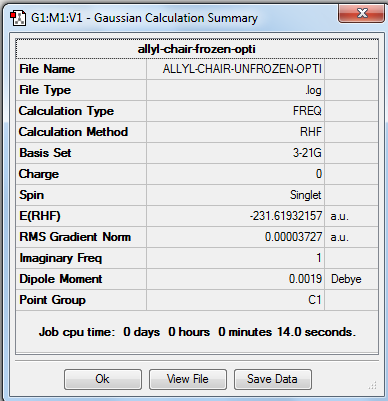
| Chair | Optimisation to a TS (Berny) | HF | 3-21G | Default | -231.61932157 a.u. | C2h |
8. Comparison to (b)
| Bond forming/breaking distances (b) | Bond forming/breaking distances (d) |
|---|---|
| 2.02026 Å | 2.02057Å |
The optimised bond distance of transition state using the redundant coordinate editor is just slightly lower than that of using computing the force constants.
(e) Optimisation of boat transition state using QST2 method
First optimisation from optimised anti2 1,5-hexadiene
1. Optimising boat transition state from optimised anti2 1,5-hexadiene
 |
 |
|---|
The correct boat structure was not obtained from the QST2 method . Hence the structure shown below which looks a bit like the chair transition state was used instead.
Second optimisation from modified reactant and product
1. Optimising boat transition state from modified reactant and product
 |
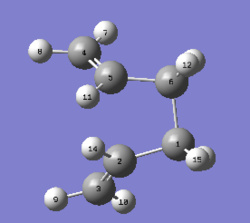 |
|---|
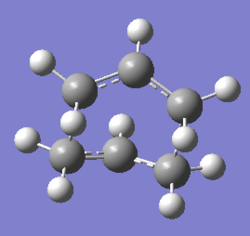 |
 |
|---|
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000066 0.000450 YES
RMS Force 0.000014 0.000300 YES
Maximum Displacement 0.000719 0.001800 YES
RMS Displacement 0.000188 0.001200 YES
Predicted change in Energy=-5.651889D-08
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
The point group of the optimised structure is C2v, confirming the structure is correct.
6. Thermochemistry
Sum of electronic and zero-point Energies= -231.450924 Sum of electronic and thermal Energies= -231.445295 Sum of electronic and thermal Enthalpies= -231.444351 Sum of electronic and thermal Free Energies= -231.479769
7. Key information
| Transition state type | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
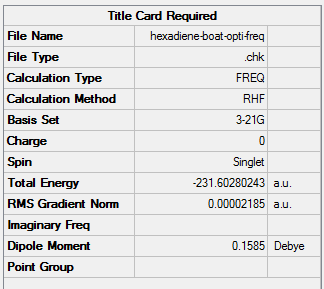
| Boat | Optimisation to a TS (QST2)+freq | HF | 3-21G | Default | -231.60280243 a.u. | C2v |
(f) IRC analysis of optimised chair and boat transition states
IRC analysis of optimised chair transition state
1. Calculating minimum energy path from chair transition state
As the reaction coordinate is symmetrical in the cope rearrangement, "forward only" is chosen for this IRC calculation. There are 44 intermediate geometries obtianed, which are connected together to show the geometric change following the calculated minimum energy path from the boat transition structure to either reactant or product.
2. General information of the first&last point of the IRC calculation
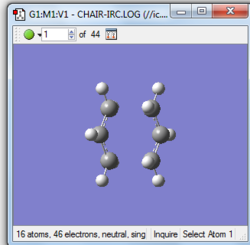 |
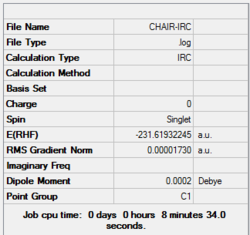 |
|---|
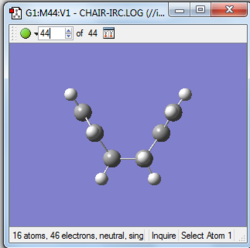 |
 |
|---|
The gradient is less than 0.001, which means the optimisation is complete.
3. Symmetry information of the last point of the IRC calculation
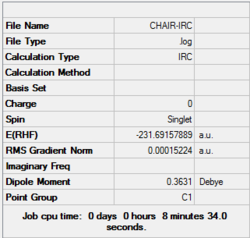
4. Key information of the IRC calculation
| Transition state type | Job type | Method | Basis set | Memory limit | Energy of the last point | Point group of the last point |
|---|---|---|---|---|---|---|
| Chair | IRC, forward only, calculate always, compute 50 points | HF | 3-21G | Default | -231.69157889 a.u. | C2 |
5. IRC plot of the IRC calculation
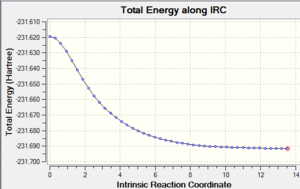 |
 |
|---|
Further optimisation
1. Optimising the last point of the IRC calculation using HF/3-21G
The structure looks almost the same as the one before optimisation.
The gradient is less than 0.001, which means the optimisation is complete. And the energy is the minimum I found, which is only slightly lower than that before optimisation.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000010 0.000450 YES
RMS Force 0.000003 0.000300 YES
Maximum Displacement 0.000300 0.001800 YES
RMS Displacement 0.000091 0.001200 YES
Predicted change in Energy=-2.408598D-09
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
5. Key information
| Transition state type | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
| Chair | Optimisation to a minimum | HF | 3-21G | Default | -231.69166702 a.u. | C2 |
Questions
Which conformers of 1,5-hexadiene do you think they connect?

The structure above is the gauch2 conformation that connect chair transition state to the boat as it's the last point of IRC pathway.
IRC analysis of optimised boat transition state
1. Calculating minimum energy path from boat transition state
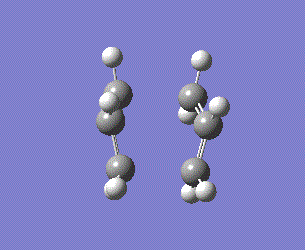
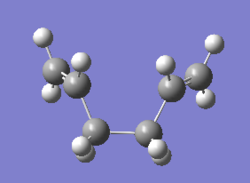
As the reaction coordinate is symmetrical in the cope rearrangement, "forward only" is chosen for this IRC calculation. There are 45 intermediate geometries obtianed, which are connected together to show the geometric change following the calculated minimum energy path from the boat transition structure to either reactant or product.
2. General information of the first&last point of the IRC calculation
 |
 |
|---|
 |
 |
|---|
The gradient is less than 0.001, which means the optimisation is complete.
3. Symmetry information of the last point of the IRC calculation
4. Key information of the IRC calculation
| Transition state type | Job type | Method | Basis set | Memory limit | Energy of the last point | Point group of the last point |
|---|---|---|---|---|---|---|
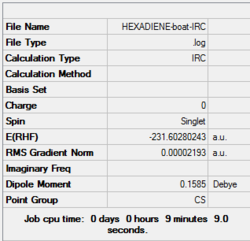
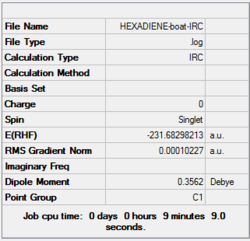
| Boat | IRC, forward only, calculate always, compute 50 points | HF | 3-21G | Default | -231.68298213 a.u. | Cs |
5. IRC plot of the IRC calculation
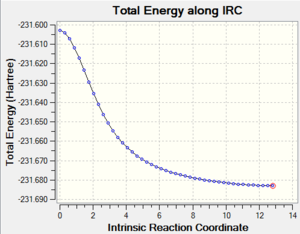 |
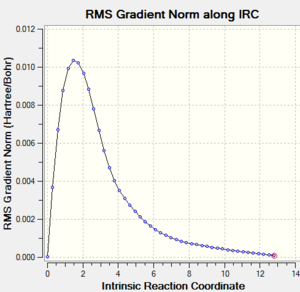 |
|---|
Further optimisation
1. Optimising the last point of the IRC calculation using HF/3-21G
The structure looks almost the same as the one before optimisation.
The gradient is less than 0.001, which means the optimisation is complete. And the energy is the minimum I found, which is only slightly lower than that before optimisation.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000026 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000402 0.001800 YES
RMS Displacement 0.000112 0.001200 YES
Predicted change in Energy=-3.711368D-09
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
5. Key information
| Transition state type | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
| boat | Optimisation to a minimum | HF | 3-21G | Default | -231.68302550 a.u. | Cs |
Questions
Which conformers of 1,5-hexadiene do you think they connect?

The structure above is the gauch5 conformation that connect boat transition state to the chair as it's the last point of IRC pathway.
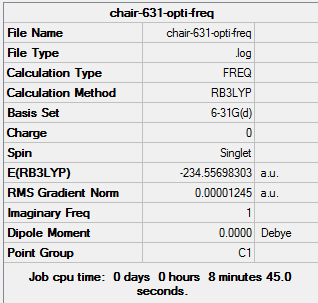
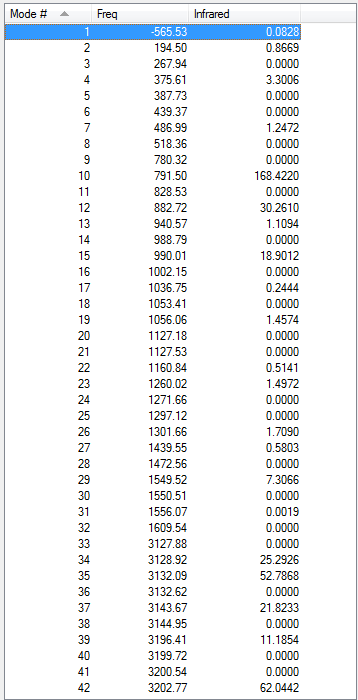
(g) Reoptimisation of chair and boat transition states using B3LYP/6-31G(d)
Reoptimisation of chair transition state
1. Optimising chair transition state using B3LYP/6-31G(d)
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000027 0.000450 YES
RMS Force 0.000005 0.000300 YES
Maximum Displacement 0.000108 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.281366D-09
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
The point group of the optimised structure is C2h, confirming the structure is correct.
6. Thermochemistry
Sum of electronic and zero-point Energies= -234.414929 Sum of electronic and thermal Energies= -234.409008 Sum of electronic and thermal Enthalpies= -234.408064 Sum of electronic and thermal Free Energies= -234.443814
7. Key information
| Transition state type | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
| Chair | Optimisation to a TS (Berny), calculate the force constants once | B3LYP | 6-31G(d) | Default | -234.55698303 a.u. | C2h |
Reoptimisation of boat transition state
1. Optimising boat transition state using B3LYP/6-31G(d)
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000018 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000695 0.001800 YES
RMS Displacement 0.000159 0.001200 YES
Predicted change in Energy=-3.028451D-08
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
The point group of the optimised structure is C2v, confirming the structure is correct.
6. Thermochemistry
Sum of electronic and zero-point Energies= -234.402339 Sum of electronic and thermal Energies= -234.396006 Sum of electronic and thermal Enthalpies= -234.395061 Sum of electronic and thermal Free Energies= -234.431749
7. Key information
| Transition state type | Job type | Additional keywords | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|---|
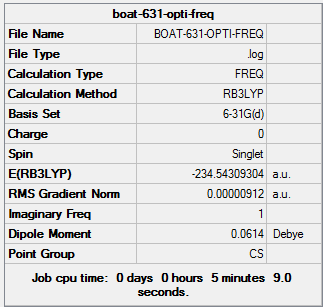
| Boat | Optimisation to a TS (Berny), calculate the force constants once | Opt=NoEigen | B3LYP | 6-31G(d) | Default | -231.54309304 a.u. | C2v |
Comparison of 3-21G and 6-31G(d) optimised reactant and transition state structures
The table below showes the energies of reactants and transition states for 2 different calculation methods:3-21G and 6-31G(d).
| HF/3-21G | B3LYP/6-31G(d) | |||||
|---|---|---|---|---|---|---|
| Electronic energy | Sum of electronic and zero-point energies (0 K) | Sum of electronic and thermal energies (298.15 K) | Electronic energy | Sum of electronic and zero-point energies (0 K) | Sum of electronic and thermal energies (298.15 K) | |
| Chair TS | -231.619322 | -231.466700 | -231.461340 | -234.556983 | -234.414929 | -234.409008 |
| Boat TS | -231.602802 | -231.450924 | -231.445295 | -234.543093 | -234.402339 | -234.396006 |
| Reactant (anti2) | -231.692535 | -231.539540 | -231.532567 | -234.611710 | -234.469204 | -234.461857 |
The activation energy for the Cope Rearrangement was calculated using Ea= ETS-Er at 0 K and 298.15 K. These values are then compared to experimentally determined activation energies given in lab script.
| HF/3-21G | B3LYP/6-31G(d) | Experimental | |||
|---|---|---|---|---|---|
| 0 K | 298.15 K | 0 K | 298.15 K | 0 K | |
| ΔEa Chair | 45.71 | 44.70 | 34.06 | 33.16 | 33.5 ± 0.5 |
| ΔEa Boat | 55.61 | 54.76 | 41.96 | 41.20 | 44.7 ± 2.0 |
The activation energies calculated for both the chair and boat conformations using 6-31G(d) method have higher accuracy as they are less different compared to the experimental values. From the table we can see chair conformation has lower activation energy and so the reaction proceeds through this conformation. Bond formation is concerted from animation of the imaginary frequency. Dotted lines are shown for 6 bonds indicating aromatic character.
The Diels Alder Cycloaddition
Diels Alder Reaction Between Cis-Butadiene and Ethylene
Optimising the Reactants
(a) Optimisation of cis-butadiene
1. Optimising cis butadiene using AM1 method
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000159 0.000450 YES
RMS Force 0.000051 0.000300 YES
Maximum Displacement 0.000783 0.001800 YES
RMS Displacement 0.000254 0.001200 YES
Predicted change in Energy=-1.540843D-07
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
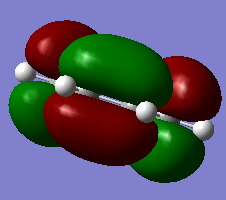
5. HOMO/LUMO visialisation
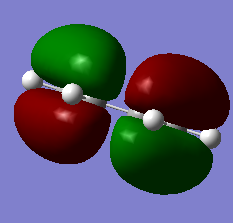 |
 |
|---|
6. Key information
| Molecule | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
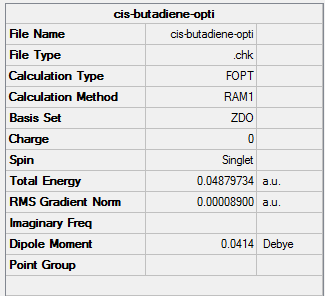
| Cis-butadiene | Optimisation to a minimum | Semi-empirical molecular orbital, AM1 | ZDO | Default | 0.04879734 a.u. | C2v |
(b) Optimisation of ethylene
1. Optimising ethylene using AM1 method
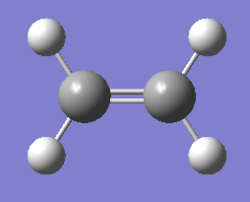
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000031 0.000450 YES
RMS Force 0.000012 0.000300 YES
Maximum Displacement 0.000057 0.001800 YES
RMS Displacement 0.000037 0.001200 YES
Predicted change in Energy=-2.644693D-09
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
5. Key information
| Molecule | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
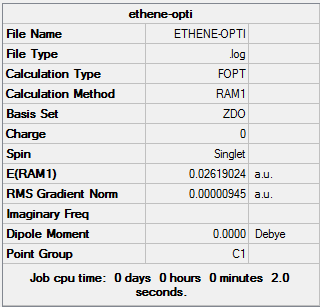
| Ethylene | Optimisation to a minimum | Semi-empirical molecular orbital, AM1 | ZDO | Default | 0.02619024 a.u. | D2h |
Optimising the Transition Structure
(a) Optimisation of guess transition state
1. Optimising guess transition state using AM1 method

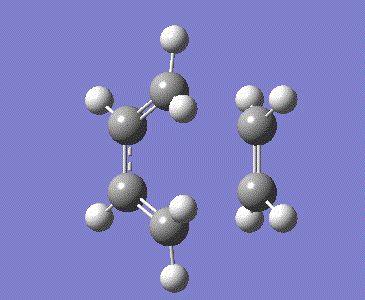
The guess transition state was drawn as above by combining the optimised ethylene and butadiene structures with two partially formed C-C bonds of 2.2 Å bond length and modifying the H-C-H bond angles. The optimised structure is shown below, which has 2.11926 Å bond lengths for the partially formed bonds.
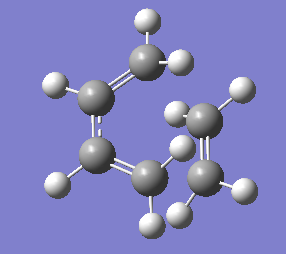
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000000 0.000015 YES
RMS Force 0.000000 0.000010 YES
Maximum Displacement 0.000000 0.000060 YES
RMS Displacement 0.000000 0.000040 YES
Predicted change in Energy=-3.424099D-17
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
6. Thermochemistry
Sum of electronic and zero-point Energies= 0.253275 Sum of electronic and thermal Energies= 0.259453 Sum of electronic and thermal Enthalpies= 0.260397 Sum of electronic and thermal Free Energies= 0.224015
7. HOMO/LUMO visialisation
 |
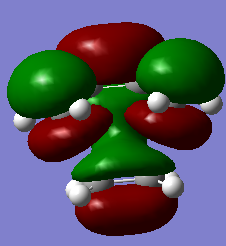 |
|---|
8. Key information
| Subject | Job type | Additional keywords | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|---|
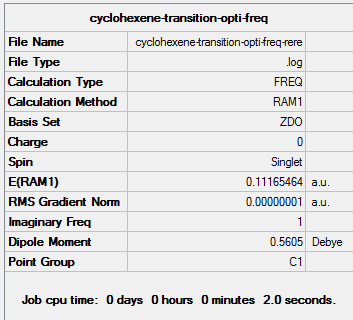
| Transition state | Optimisation to a TS (Berny), calculate the force constants always | Opt=NoEigen | Semi-empirical molecular orbital, AM1 | ZDO | Default | 0.11165464 a.u. | Cs |
(b) IRC analysis of optimised transition state
1. Calculating minimum energy path from transition state
As the reaction coordinate is not symmetrical in the Diels Alder cycloaddition, "both directions" is chosen for this IRC calculation. There are 87 intermediate geometries, which are connected together to show the geometric change following the calculated minimum energy path from reactant to product via the transition state. The structure of the last point of this IRC calculation is shown below.
2. IRC plot
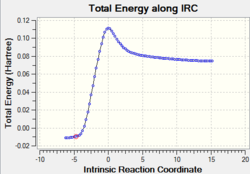 |
 |
|---|
As the IRC plot is shown above, the energy minimum is reached in this calculation because the RMS gradient reaches 0 in the end. Therefore no need to conduct further calculation. The general and symmetry information of the last point of this IRC calculation is given in the following.
The gradient is less than 0.001, which means the optimisation is complete.
5. Key information
| Subject | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
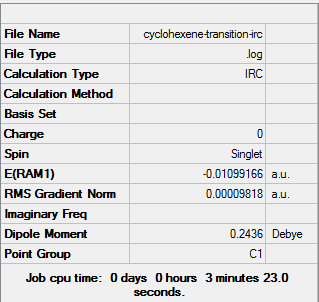
| Transition state | IRC, both directions, calculate always, compute 100 points | Semi-empirical molecular orbital, AM1 | ZDO | Default | -0.01099166 a.u. | Cs |
Discussion
| sp3 C-C | sp2 C=C | sp3-sp2 C-C | van der Waals radius of C | partly formed σ C-C bond |
| 1.52 | 1.33 | 1.50 | 1.70 | 2.12 |
The table above shows literature values of different C-C bond lengths. The bond length calculated from optimisation of transition state is shorter than two van der Waals radii which shows attractive forces between terminal carbons of cis-Butadiene and ethylene. In addition, the bond distanced is much larger than any of the literature values, indicating the bond is only partly formed.
Diels Alder Reaction Between Cyclohexa-1,3-diene and Maleic Anhydride
Optimising the Reactants
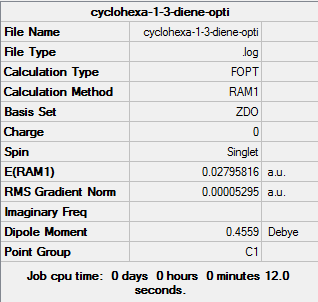
(a) Optimisation of cyclohexa-1,3-diene
1. Optimising cyclohexa-1,3-diene using AM1 method

The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000149 0.000450 YES
RMS Force 0.000031 0.000300 YES
Maximum Displacement 0.001024 0.001800 YES
RMS Displacement 0.000279 0.001200 YES
Predicted change in Energy=-2.196587D-07
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
5. Key information
| Molecule | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
| Cyclohexa-1,3-diene | Optimisation to a minimum | Semi-empirical molecular orbital, AM1 | ZDO | Default | 0.02795816 a.u. | C2v |
(b) Optimisation of maleic anhydride
1. Optimising maleic anhydride using AM1 method
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000129 0.000450 YES
RMS Force 0.000051 0.000300 YES
Maximum Displacement 0.001415 0.001800 YES
RMS Displacement 0.000439 0.001200 YES
Predicted change in Energy=-3.063481D-07
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
5. Key information
| Molecule | Job type | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|
| Maleic anhydride | Optimisation to a minimum | Semi-empirical molecular orbital, AM1 | ZDO | Default | -0.12182404 a.u. | C2v |
Optimising the Exo and Endo Transition Structures
(a) Optimisation of Exo transition state
1. Optimising exo transition state using AM1 method
The guess exo transition state was drawn as above by combining the optimised cyclohexa-1,3-diene and maleic anhydride structures with two partially formed C-C bonds of 2.2 Å bond length and modifying the cyclohexa-1,3-diene into envelope structre. The optimised structure is shown below, which has 2.17078 Å bond lengths for the partially formed bonds.
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000000 0.000015 YES
RMS Force 0.000000 0.000010 YES
Maximum Displacement 0.000001 0.000060 YES
RMS Displacement 0.000000 0.000040 YES
Predicted change in Energy=-4.648033D-15
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
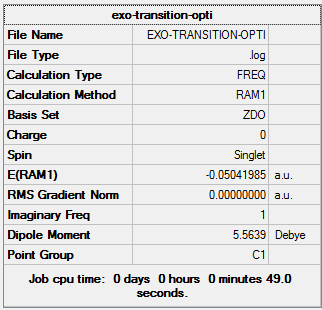
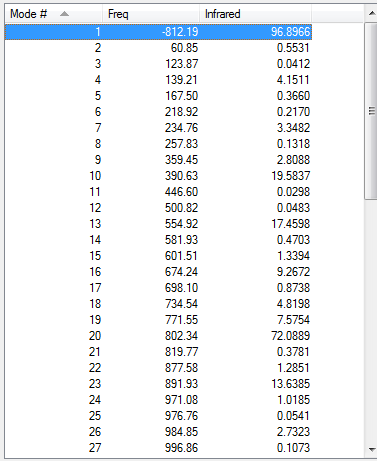
As the above table is shown, there is only one imaginary frequency that has a magnitude of 812.23 cm-1. The corresponding vibrational mode is shown below. Although the reactants different, the transition structure for reaction between cyclohexa-1,3-diene and maleic Anhydride has a similar vibrational motion as the transition state structure for reaction between cis-Butadiene and ethylene obtained earlier, that is, the two C atoms of maleic Anhydride and the two middle C atoms of cyclohexa-1,3-diene approach each other in a sychronised motion and facilitates two simultaneous C-C bonds formation.
6. Thermochemistry
Sum of electronic and zero-point Energies= 0.134881 Sum of electronic and thermal Energies= 0.144881 Sum of electronic and thermal Enthalpies= 0.145826 Sum of electronic and thermal Free Energies= 0.099118
7. Key information
| Transition state type | Job type | Additional keywords | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|---|
| Exo | Optimisation to a TS (Berny), calculate the force constants always | Opt=NoEigen | Semi-empirical molecular orbital, AM1 | ZDO | Default | -0.05041985 a.u. | Cs |
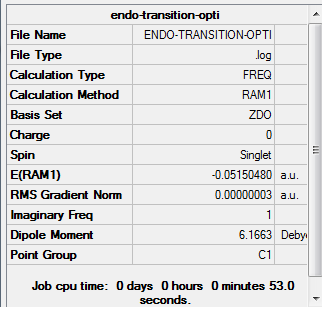
(b) Optimisation of Endo transition state
1. Optimising endo transition state using AM1 method
The guess endo transition state was drawn in a similar way as for exo transition state.
The gradient is less than 0.001, which means the optimisation is complete.
3. Real output
Item Value Threshold Converged?
Maximum Force 0.000000 0.000015 YES
RMS Force 0.000000 0.000010 YES
Maximum Displacement 0.000009 0.000060 YES
RMS Displacement 0.000002 0.000040 YES
Predicted change in Energy=-1.103715D-12
Optimization completed.
-- Stationary point found.
Both force and displacement are converged, indicating the success of optimisation.
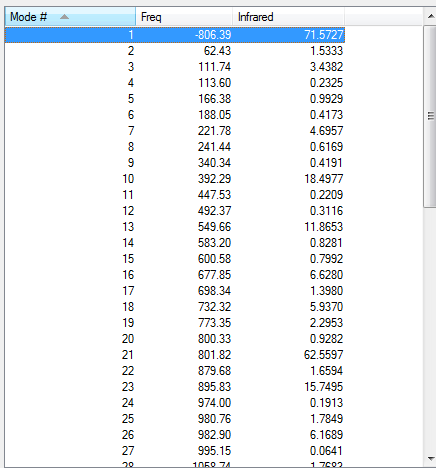
As the above table is shown, there is only one imaginary frequency that has a magnitude of 806.40 cm-1. The corresponding vibrational mode is shown below, and the endo transition structure has a similar vibrational motion as the exo transition structure obtained earlier.
6. Thermochemistry
Sum of electronic and zero-point Energies= 0.133494 Sum of electronic and thermal Energies= 0.143683 Sum of electronic and thermal Enthalpies= 0.144628 Sum of electronic and thermal Free Energies= 0.097350
8. Key information
| Transition state type | Job type | Additional keywords | Method | Basis set | Memory limit | Energy | Point group |
|---|---|---|---|---|---|---|---|
| Endo | Optimisation to a TS (Berny), calculate the force constants always | Opt=NoEigen | Semi-empirical molecular orbital, AM1 | ZDO | Default | -0.05150480 a.u. | Cs |
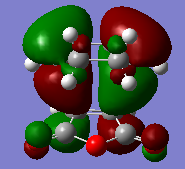
(c) HOMO visialisation of exo and endo transition state
 |
 |
|---|
Discussion
The activation energy of exo transition state is 27.26281829 kcal/mol, which is higher than that of endo transition state(26.58200131 kcal/mol). Hence endo transition state is more stable than the exo one leading to major product being endo. This is because secondary orbital overlap effects- the HOMO of the butadiene fragment has the right phase to interact with LUMO of the anhydride fragment, stabilising the endo transition state. There is node between butadiene and anhydride fragment in exo transition state hence there is no interaction between them.