Rep:Mod:1299
Properties of NH3
Summary of optimisation results
Calculation method: RB3LYP
Basis set:6-31G(d,p)
Final energy:-56.55776873 au
RMS gradient:0.00000485 au
Point group: C3V
N-H bond length:1.018 Å
H-N-H bond angle:106°
| Item | Value | Threshold | Converged |
|---|---|---|---|
| Maximum Force | 0.000004 | 0.000450 | YES |
| RMS force | 0.000004 | 0.000300 | YES |
| Maximum Displacement | 0.000072 | 0.001800 | YES |
| RMS Displacement | 0.000035 | 0.001200 | YES |
NH3 |
Vibrational and frequency analysis
Fig. 1: The display vibrations window for NH3
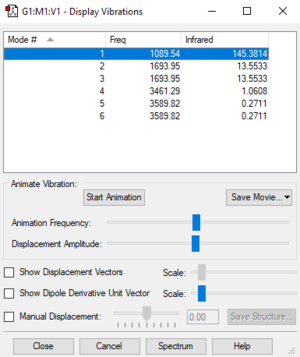
| Wavenumber cm-1 | 1090 | 1694 | 1694 | 3461 | 3589 | 3589 |
| Symmetry | A1 | E | E | A1 | E | E |
| Intensity arbitrary units | 145 | 14 | 14 | 1 | 0 | 0 |
| Image |  |
 |
 |
 |
 |
|
From the 3N-6 rule, we could expect 3 vibrational modes (3*3-6=3). As it appears we have 6 modes, there must be multiple degenerate modes. We can see from the .log file and the "display vibrations" window that we have 2 pairs of degenerate vibration modes. They are the second and third (1694 cm-1) and fifth and sixth (3590 cm-1) in the table. From the table, we can see that the first three modes (1090 and 1694 cm -1 are "bending" modes and the next three (3461 and 3590 cm -1) are "stretching" modes. The stretching mode at 3461 cm -1 is highly symmetric as it has three C3 axes and the symmetry does not change. We can also say that the "bending" mode at 1090 cm-1 looks like an umbrella opening and closing. From the vibrational energies, we could predict there would be two bands on a gaseous ammonia spectrum. There are only three modes with a large change in dipole moment (1090 and 1694 cm-1), two being degenerate. Due to this, we could expect a peak at 1090 cm-1 and another at 1694 cm-1.
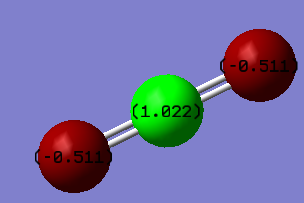
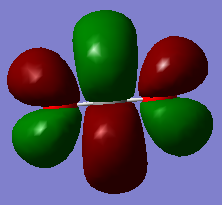
Fig.2.: Atomic charges of the ammonia molecule.
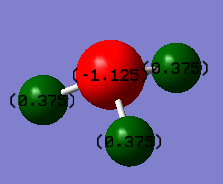
When thinking about this molecule, we would expect the nitrogen atom to have a negative charge and the hydrogens positive charges as the nitrogen is far more electronegative than the hydrogens. As anticipated, nitrogen has a charge of -1.125 and the hydrogens 0.375.
Properties of N2
Summary of optimisation results
Calculation method: RB3LYP
Basis set:6-31G(d,p)
Final energy:-109.52412868 au
RMS gradient:0.00000060 au
Point group: D*H
N-H bond length:1.09 Å
Proof of optimisation:
Item Value Threshold Converged? Maximum Force 0.000001 0.000450 YES RMS Force 0.000001 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000000 0.001200 YES
N2 |
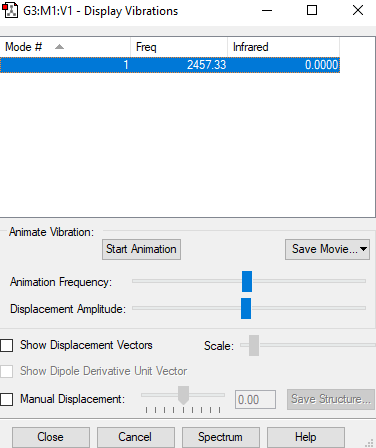
Fig.3.:: The display vibrations table for N2
| Wavenumber cm-1 | 2457 |
| Symmetry | D*H |
| Intensity arbitrary units | 12 |
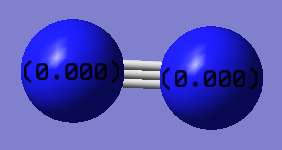
Fig.4.: Atomic charges of N2
Properties of H2
Summary of optimisation results
Calculation method: RB3LYP
Basis set:6-31G(d,p)
Final energy:-1.17853936 au
RMS gradient:0.00000017 au
Point group: D*H
H-H bond length:0.75 Å
Proof of optimisation:
Item Value Threshold Converged? Maximum Force 0.000000 0.000450 YES RMS Force 0.000000 0.000300 YES Maximum Displacement 0.000000 0.001800 YES RMS Displacement 0.000001 0.001200 YES
H2 |
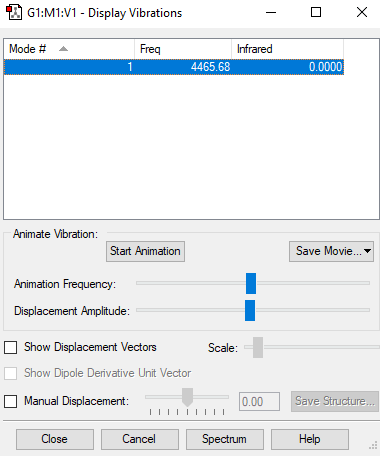
Fig.3.:: The display vibrations table for H2
| Wavenumber cm-1 | 4466 |
| Symmetry | D*H |
| Intensity arbitrary units | 12 |
Fig.4.: Atomic charges of H2
H2 in larger structures
H-H bonds are present in a number of structures such as CEFCAS. The optimised bond distance reported by Gaussian is 0.75 Å whereas the bond length in the CEFCAS structure is 1.482 Å. This is up to a few reasons. First of all, the hydrogen atoms in the large structure are in a 3 centre-2 electron configuration where there is a sort of delocalisation of electrons allowing the formation of 2 bonds from each hydrogen. This results in lower electron density, a weaker bond and subsequently, a longer bond. Furthermore, this molecule is composed of a large variety of atoms and structures, all with some effect on the bond lengths, such as the phenyl group with their characteristic delocalisation. Another reason for this would be that the H-H bond in the H2 is done computationally, resulting in a value somewhat devoid of any real-world considerations such as interaction with other molecules or forces.
A look at the Haber-Bosch process
Energies of formation
E(NH3)=-56.5577687 au
2*E(NH3)=-113.1155374 au
E(N2)=109.5241286 au
E(H2)=-1.1785393 au
3*E(H2)=-3.5356179 au
ΔE=2*E(NH3)-[E(N2)+3*E(H2)]=-0.0557909 au
or in the usual units ΔE=-146.4790079 kJ/mol
As the reaction has a negative energy difference, we can say that it is exothermic and as a result, the equilibrium lies on the side of the ammonia product.
Properties of CO2
Summary of optimisation results
Calculation method: RB3LYP
Basis set:6-31G(d,p)
Final energy:-188.5809395 au
RMS gradient:0.00001154 au
Point group: D∞H
N-H bond length:1.169 Å
Proof of optimisation:
Item Value Threshold Converged? Maximum Force 0.000024 0.000450 YES RMS Force 0.000017 0.000300 YES Maximum Displacement 0.000021 0.001800 YES RMS Displacement 0.000015 0.001200 YES
CO2 |
Vibrational and frequency analysis
Fig. 5: The display vibrations window for CO2

| Wavenumber cm-1 | 640 | 640 | 1372 | 2436 |
| Symmetry | PIU | PIU | SGG | SGU |
| Intensity arbitrary units | 31 | 31 | 0 | 546 |
| Image |  |
 |
 |
|
We can see from the vibrational information that there are only three infrared and Raman active modes. This is due to the third mode in the table not having a change in dipole moment. However, there would only be two distinct peaks on the spectrum because the first two modes are degenerate. This is because they vibrate in the same way, the difference being that one moves in the plane, the other in and out of it.
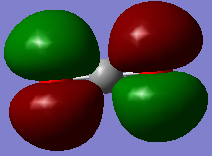
Fig. 6.: Atomic charges of CO2
The partial negative charge on the two oxygen atoms is due to the fact they are much more electronegative than the carbon atom. As such, they pull electron density away from the carbon.
Molecular orbital analysis
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have received your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 0.5/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
YES
Have you included answers to the questions about vibrations and charges in the lab script?
YES - However, for the calculation on expected band you used N=3 for ammonia but it actually is a molecule consisting of 4 atoms (N=4). You are stating the ammonia in the vibration at 3461cm-1 to have 3 C3 axes but it has only one C3 axis which remains during the vibration.
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
NO - You missed to include a link to the .log file of your finished calculation. This reduces the achievable mark for this section by 1.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES
Haber-Bosch reaction energy calculation 0.5/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
NO - Energies in kJ/mol should be reported to one digital place only.
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 3/5
Have you completed the calculation and included all relevant information?
NO - You missed to include a link to the .log file of your finished calculation. This reduces the achievable mark for this section by 1.
Have you added information about MOs and charges on atoms?
YES
Your discussion on the computed vibrations is good. In future reports try to be more precise with the meaning in-plane and out-of- plane. For CO2 you can define an indefinite number of planes but for this instance this will not be penalised.
You could have reported the occupancy of the MOs for all displayed ones as well as stating the contributing AOs in all cases. For the first three MOs you got confused about the meaning of the MO colours. The two different colours depict the two different phases of the MOs and are not directly related to bonding and anti-bonding properties. The first three MOs are all non-bonding as no overlapping AOs are observed.
Independence 0/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or Do some deeper analysis on your results so far
NO- no independent work ha been identified.