Rep:Mod:1012017
Transition States and reactivity
Introduction
A potential energy surface is to describe how energy changes with molecular geometries. A PES has many dimensions (3N-6) , where N is the number of atoms in molecules but usually 3 dimensions are required for atom coordinates.

Nf710 (talk) 22:22, 29 November 2017 (UTC) It is degrees of freedom not nuclear coordinates. nuclear coordinates leads to linear dependancies in the basis set.
In a PES, reactants and products are minimum points whereas the transition state corresponds to a local maximum(saddle) point. For both of minimum points and saddle points on PES, the first derivatives are 0. Therefore in order to distinguish between them, calculations for second derivatives are required as second derivatives measure the curvature at stationary points. Minimum points will have positive second derivatives and at minimum points any changes in geometry will lead to an increase in energy. Transition states will have negative second derivatives and the energy only decreases in the reaction path way direction.
Nf710 (talk) 22:24, 29 November 2017 (UTC) TSs have positive curvature in all dimensions apart from 1 which has negative, which is the reaction coordinate.

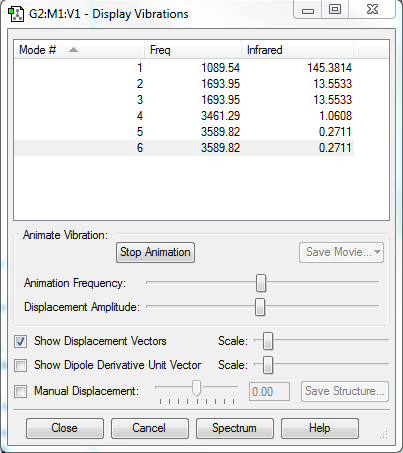
Non-linear molecules have 3N-6 different vibration modes, each of the vibration modes has an associated force constant. The force constant is calculated by the second derivatives on PES then frequencies can be calculated using reduced mass. Hence various rotational and translational modes will be separated out from the real vibrational modes. As force constants are negative values thus frequencies calculated by force constants are imaginary frequencies. Optimisaition calculations should only obtain 1 imaginary frequency, which indicates that the optimised structure is transition state and all real vibrational frequencies confirm the structure is minimum. [1]
Nf710 (talk) 22:25, 29 November 2017 (UTC) This 1 imaginary vibration comes from the curvature being negative in old one of the 3N-6 dimensions of the PES at the TS.
Method
For all of three reactions, transition states were found by starting optimisation with products. The structure of products were drawn and then were optimised to minimum at semi-empirical PM6 level. Calculated frequencies were checked to ensure no negative frequency at this stage.(symmetry of input structure may require to adjust to break the symmetry) Then the bonds that would form during the reaction were broken( i.e. C-O, C-S in reaction of xylylene and SO2 ). The separation between the broken bonds were set to certain values and coordinates of atoms that correspond to the broken bonds were fixed. (i.e. C-C=2.2 Å, C-O=2.0 Å, C-S=2.4 Å) Then the structure was optimised to minimum at PM6 level again to get a closed transition state structure. The structure was further optimised to transition state under different basis set. If BY3LYP/631g(d) method is required, the structure was optimised to PM6 level first and then further optimised to B3YLYP level using Density Function Theory ((DFT). After optimisation, the structure should have an imaginary frequency and IRC calculation was carried out to ensure correct transition state was obtained by inspecting the general shape of IRC and the transition state should have a zero gradient on IRC. The desired products were determined from the IRC calculation and further optimised to a more accurate geometry at minimum. Activation energies and reaction energies were calculated for Exercise 2 and 3 to find out the kinetically/thermodynamically products.
Results and Discussion
Exercise 1: Reaction of Butadiene with Ethylene
Optimisation
| Ethlyene | Butadiene | Transition State | Product | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Transition State frequency and IRC analysis
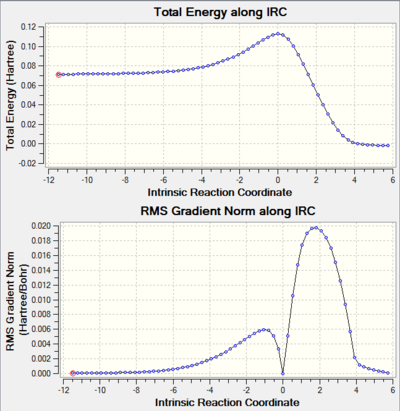
Only one imaginary frequency was obtained after frequency calculation, which proves the formation of single transition state. Imaginary frequency is the second derivatives of the potential energy curve therefore if the output structure is a transition state( maximum in energy) then the first derivatives should be zero and the second derivatives will be less than zero to indicate that the maximum point was found. Also the gradient =0 at IRC=0 in RMS gradient Norm along IRC graph confirms the formation of transition state.
(Fv611 (talk) You didn't include the discussion about the timing of the bond formation. Is it synchronous or asynchronous?)
 |
 |
 |
 |
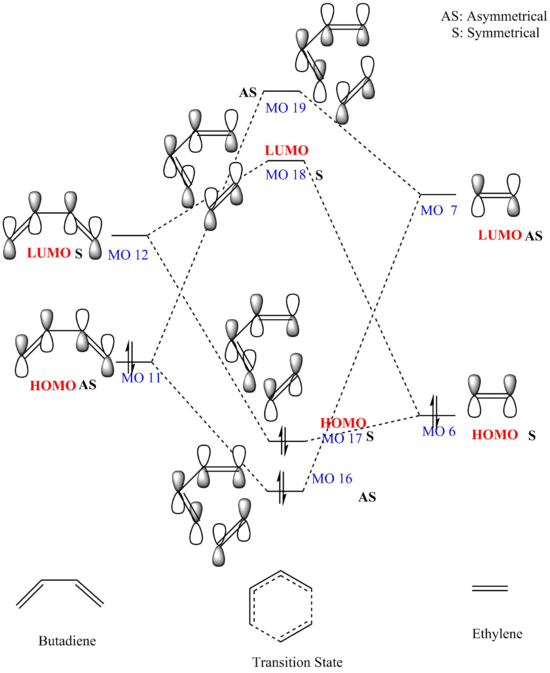
MO diagram Analysis
- In order to from MOs, orbitals only combine with those have the same symmetry to have non-zero overlap integrals thus asymmetrical orbitals with symmetrical orbitals will have zero overlap integrals. Molecules are impossible to react if the overlap integral is zero therefore symmetries of interacting MOs have to match with each other.

In Figure 2, asymmetrical HOMO (MO 11)of butadiene combines with asymmetrical LUMO (MO 7) of ethylene and forms asymmetrical HOMO (MO 16) and LUMO (MO 19) of the transition state .Symmetrical LUMO orbital of butadiene combines with symmetrical LUMO (MO 6) orbital of ethylene and forms symmetrical MO 17 and MO 18 orbitals of the transition state.
- Woodward-Hoffmann Rules state that in a thermal pericyclic reaction, the sum of (4q+2)s and (4ra) must be odd. (4q+2)s+(4r)a refers to the number of electrons present in the components and q,r are any integers from 0. "s" stands for suprafacial, which means new bonds form on the same side at both ends . "a" stands for antarafacial and means new bonds from on opposite sides at both ends. Butadiene reacts with ethylene via [π4s+π2s] cycloaddition therefore there is no (4r)a component.
(4q+2)s+(4r)a =1 therefore thermally allowed
- Ideally, the high energy HOMO of electron rich diene will give a better overlap ( large splitting )with the low energy LUMO of electron poor dienophile as they have small energy gap. However, in the thermal [4+2] cycloaddition, mixing occurs for both HOMO and LUMO.
(Fv611 (talk) Your MO diagram combinations are correct, but your energy levels are not. You have calculated the MOs with the correct energies, so why not adjust your MO diagram accordingly? For example, you have calculated the energy of the Ethene HOMO to be -0.39, and that of the TS HOMO-1 to be -0.32, and yet you draw the TS HOMO-1 as more stable than the Ethene HOMO. The same sort of mistake was made in the LUMOs too. Additionally, you haven't labeled the MOs consistently between the Jmols and the diagram.)
Bond Length Analysis
| sp3 C- sp3 C | sp2 C- sp2 C | sp3 C- sp2 C | VDW radius of C | |
|---|---|---|---|---|
| Typical Bond Length/Å | 1.54 | 1.47 | 1.50 | 1.70 |

In the transition state, C12-C10 becomes shorter than a standard sp2 C- sp2 C bond and (C12-C14,C1-C4,C10-C17) lengthen. This is because C12-C10 forms a partial double bond and (C12-C14,C1-C4,C10-C17) start to loose pi-pi overlap. Carbon atoms in the double bond change hybridisation from sp2 to a sp3-like in the transition state therefore the % s character of C atom decrease from 33% (sp2) to 25% (sp3). As electrons in s orbitals are held more closely to the nucleus than electrons in p orbitals therefore atoms that have more s characters will have shorter bond length. Bond lengths of C4-C14 and C1-C7 are between sp3-sp3 and Van Der Waals distance of C-C (3.4 Å), which indicates bond formation.
| Bond | Bond Length Vs IRC | Discussion |
|---|---|---|
| C1-C4 | 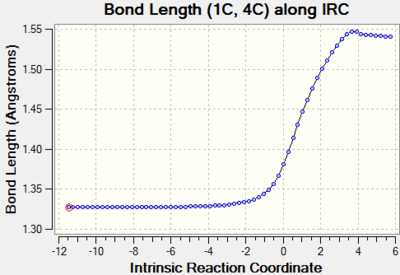 |
Bond order of these three bonds changes from double bond to single bond and their bond lengths all increase through reaction path. This is because double bond breaks and changing in hybridisation lengthens the bond. |
| C12-C14 | 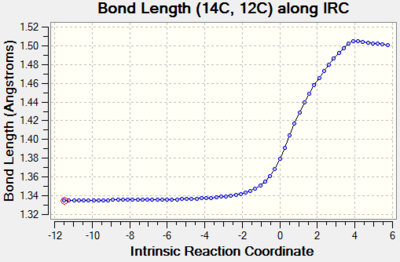 | |
| C10-C17 | 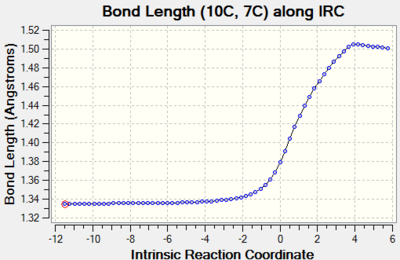 | |
| C10-C12 | 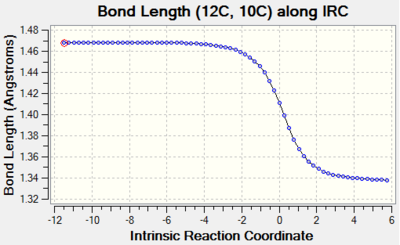 |
Bond order of C10-C12 changes from single bond to double bond and the bond length decreases through reaction path. This is due to the formation of strong C-C double bond that can hold the electrons closely to the nucleus. |
| C4-C14 |  |
Distances between C4-C14 and C1-C7 are initially VDW distances for C-C atom. As the reaction proceeds, the distances for C4-C14, C1-C7 decreases. The changing in bond length indicates the formation of cyclohexene and the two bond length Vs IRC graphs coincide with each other also indicates the bond formation is synchronous. |
| C1-C7 | 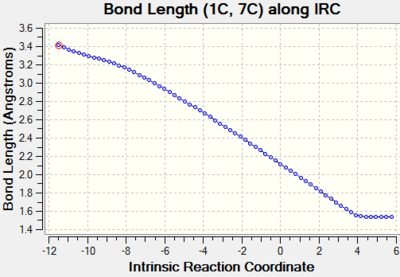 |
Exercise 2: Reaction of Cyclohexadiene and 1,3-Dioxole
Optimisation to B3LYP/6-31G Level
| Cyclohexadiene | 1,3-Dioxole | |||||
|---|---|---|---|---|---|---|
| Structure | ||||||
| Frequency analysis |  |
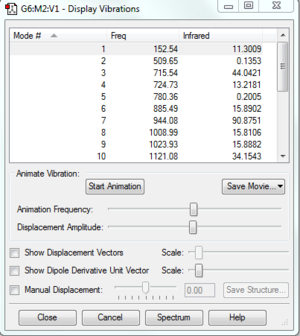
|
| Transition State | Product | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure |
|
|
|
| ||||||||||||
| Frequency analysis | 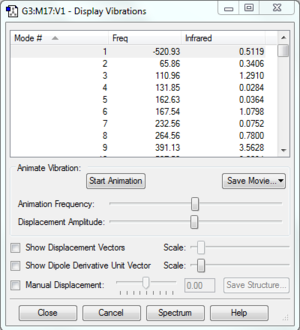 |
 |
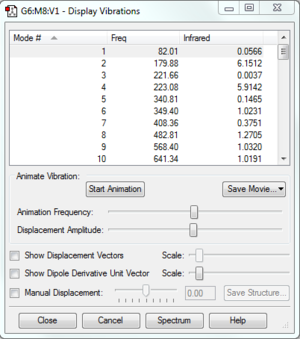 |
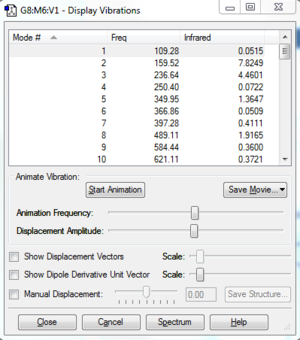 | ||||||||||||
MO Analysis
| Endo TS HOMO | Endo TS MO 40 | Endo TS LUMO | Endo TS MO 43 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
| ||||||||||||
| EXO TS HOMO | EXO TS MO 40 | EXO TS LUMO | EXO TS MO 43 | ||||||||||||
|
|
|
|
 |
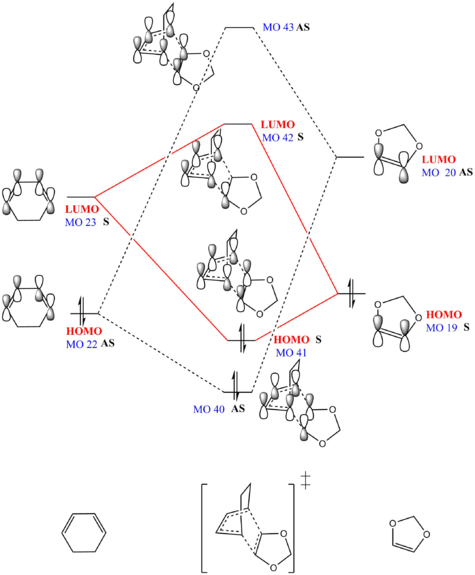 |
(Fv611 (talk) Your MO pairings are correct, but you didn't represent the TS MOs correctly according to their relative energies. Indeed the bonding TS MOs both have a higher energy than the diene's HOMO, and the antibonding TS MOs both have lower energy than the dienophile's LUMO. Well done on highlighting the difference between the exo and endo case.)
- There are two types of Diels-Alder reactions, either normal electron demand Diels-Alder reaction or inverse electron demand Diels Alder reaction. The two types of reactions are determined by the relative energy levels of frontier orbitals(highlighted in red in the MO diagram) as FMO( Frontier Molecular Orbital Theory) simplifies the reactivity of a reaction to the interactions between HOMOs and LUMOs . In a normal electron demand Diels-Alder reaction, HOMO(high energy) of electron rich diene interacts with the LUMO(low energy) of electron poor dienophile. On the contrary, for an inverse electron demand Diels-Alder reaction, LUMO ( low energy) of electron poor diene interacts with HOMO(high energy) of electron rich dienophile.[3]
- Therefore the reaction between cyclohexadiene and 1,3-dioxole is an inverse electron demand Diels-Alder reaction. As can be seen in both MO diagrams, HOMO and LUMO of 1,3-dioxole (dienophile) are higher in energy than HOMO and LUMO of cyclohexadiene(diene) unlike the orbital interaction in a normal DA reaction, HOMO of 1,3-dioxole and LUMO of cyclohexadiene are FMOs. Moreover, they have smaller energy gap thus stronger overlap and interaction. This is mainly because the lone pair electrons in p orbital on O atom increases electron density of dienophile and increases the energy levels. [4]
Nf710 (talk) 22:36, 29 November 2017 (UTC) YOu could have looked at the ordering of the orbitals in the reactants when they were on the PES at the start of the IRC
Reaction Energies and Barriers
| Structure | Energies Calculated From PM6 Level/Kjmol-1 | Energies Calculated From B3LYP/631G(d)/Kjmol-1 |
|---|---|---|
| Cyclohexadiene | +306.8501 | -6.12593 X 105 |
| 1,3-Dioxole | -137.2506 | -7.01188 X 105 |
| Endo Transition State | +362.1641 | -1.3136 X 106 |
| Exo Transition State | +364.6899 | -1.3136 X 106 |
| Endo Product | +99.254 | -1.3138 X 106 |
| Exo Product | +99.7007 | -1.3138 X 106 |
| EXO Product | PM6 | B3LYP/631G(d) |
|---|---|---|
| Activation Energy (Eact) / Kjmol-1 | + 195.1 | + 167.0 |
| Reaction Energy/ Kjmol-1 | - 69.9 | - 64.0 |
| ENDO Product | PM6 | B3LYP/631G(d) |
|---|---|---|
| Activation Energy (Eact) / Kjmol-1 | + 192.6 | + 159.0 |
| Reaction Energy / Kjmol-1 | - 70.3 | - 68.0 |
- Energy unit was converted from hartree to Kjmol-1 by dividing 3.8088 X10-4
- Activation Energy (Eact)= Energy of TS - Energies of reactants
- Reaction Energy = Energy of product - Energies of reactants
- By comparing the data calculated through B3LYP method, the endo product is 8 Kjmol-1 less positive in activation energy and 4Kjmol-1 more negative in reaction energy than the exo product. Lower activation energy means the reaction can form transition state faster and take less energy thus the reaction is kinetically favourable in this case endo product is kinetically favourable. A more negative reaction energy means that the reaction forms more stable product by releasing more heat therefore endo product is thermodynamically favourable. The driving force of the reaction is the formation of new σ-bonds, which are energetically more stable than the π-bonds.
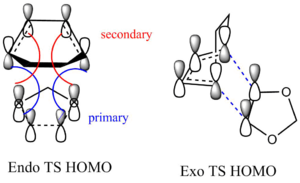
Secondary Orbital Interaction
| Endo-TS | Exo-TS | Discussion | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
The reason why Endo products are preferred in the DA reaction is because of the presence of secondary orbital interaction.(2 p orbitals that contain electron lone pairs on O atoms overlap with LUMO of diene.)
Both secondary orbital interaction and primary interaction are observed in the endo TS whereas exo TS only has primary interaction. Primary interaction directs orientation of reactants to form sigma bond but secondary orbital interaction greatly stabilizes the transition state by lowing activation energy. Therefore Endo TS has a smaller activation energy than Exo TS and Endo TS can be formed faster(kinetically favourable). The steric clash effect that presents in the exo transition state also results in a higher transition state though the contribution from steric clash is minor.
 | ||||||
 | ||||||||
Your energies are correct and you have come to the correct conclusions and you have argued them well. This is a really good section. It would have been nice if you could have spoke in more detail about the quantum chemical methods used in the intro.
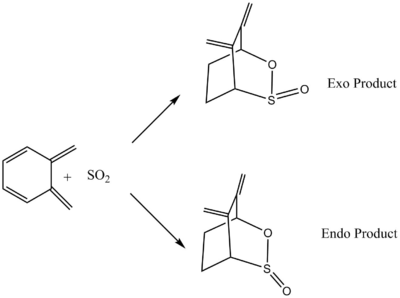
Exercise 3: Diels-Alder vs Cheletropic
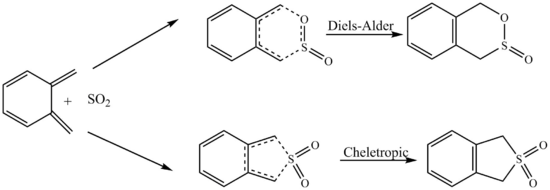
Optimisation to PM6 Level
| Xylylene | EXO TS | ENDO TS | Cheletropic TS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SO2 | Exo Product | Endo Product | Cheletropic Product | ||||||||
Visualise the reaction coordinate with an IRC calculation
| Reaction Pathway | gif | IRC |
|---|---|---|
| DA reaction EXO pathway | 
|
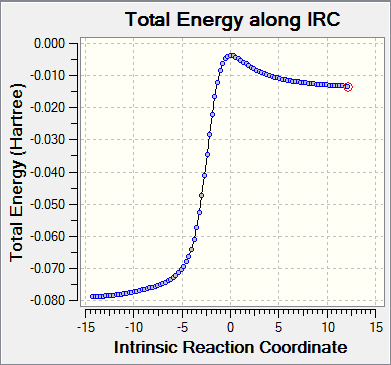 |
| DA reaction ENDO pathway | 
|
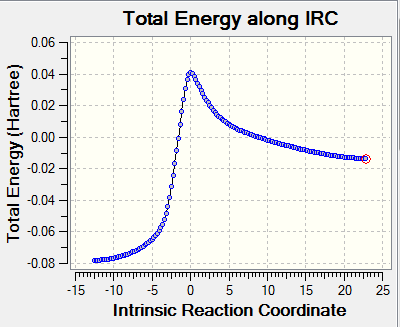 |
| Cheletropic | 
|
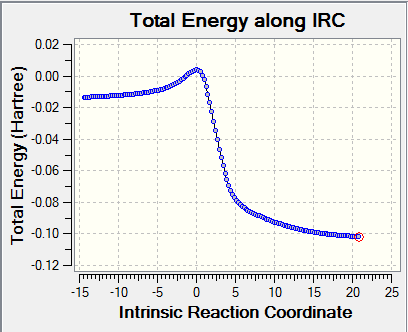
|
For Exo and Endo pathway, total energy along IRC starts from products to reactants. The graphs show an inverse of the normal reaction pathway as reactions are exothermic. It is clear to see in the animation that two alkene bonds outside the 6-membered ring system form a cyclic system first and then forming a double conjugate with the nearby ring. Thus the planar 6-membered ring is able to gain aromaticity as there are now 6 pi electrons in the the planar ring system (Huckle's rule 4n+2 pi electrons). The extra stabilities gained from formation of benzene rings in all three reactions facilitate this exothermic reaction. This can be confirmed by measuring the C-C bond length in the products of all three reactions. The C-C bond length of all C-C bonds in the 6-membered ring became 1.47 Å, which is in between C-C and C=C bond lengths. This indicates that electrons delocalised over these 6 carbon atoms.
(The reversed pathways are only because Gaussian has been given no definition of "reactant" and "product" Tam10 (talk) 11:59, 24 November 2017 (UTC))
Activation Barrier and Reaction Energy
| Structure | Exo | Endo | Cheletropic |
|---|---|---|---|
| xylylene | +467.1447 | +467.1447 | +467.1447 |
| SO2 | -311.4211 | -311.4211 | -311.4211 |
| Transition State | +241.7482 | +237.7679 | +260.3577 |
| Product | +56.3301 | +56.9760 | -0.0053 |
| Activation Barrier/kjmol-1 | +86.02 | +82.04 | +104.28 |
| Reaction Energy/kjmol-1 | -99.39 | -98.74 | -155.73 |
Reaction Profile
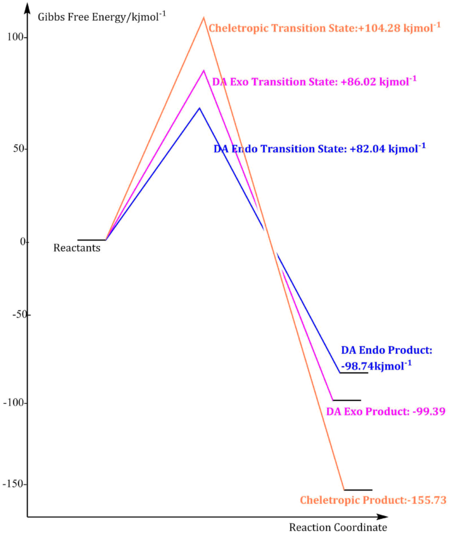
Cheletropic reaction is the most exothermic reaction among all these reactions followed by DA exo pathway and endo pathway therefore the cheletropic product is the most thermodynamically favourable and under thermodynamic control. DA endo pathway has the smallest activation energy among three reactions therefore DA endo product is the most kinetically favourable and the pathway is under kinetic control.
Extension
| Structure | Exo | Endo |
|---|---|---|
| xylylene | +467.1447 | +467.1447 |
| SO2 | -311.4211 | -311.4211 |
| Transition State | +275.8219 | +267.9874 |
| Product | +176.7067 | +172.2591 |
| Activation Barrier/Kjmol-1 | +120.10 | +112.26 |
| Reaction Energy/Kjmol-1 | +20.98 | +16.28 |
By comparing with results in Exercise 3, activation energies for both pathways are larger than the activation energies of the diene that outside the 6-membered ring. Therefore it is kinetically unfavourable to for dienophiles to react with diene inside the the 6-membered ring. Products that formed from reacting with diene that outside the 6-membered ring are the major products.
Conclusion
This experiment investigates about different pathways of Diels-Alder reaction and Cheletropic reaction by using Gaussian to take various measurements about bond lengths, activation and reaction energies and also visualize molecule orbitals to construct MO diagrams. The IRC calculation also simulates the approaching trajectories of reactants in Diels-Alder reactions, which verifies that the bond formation is synchronous. By changing the electronic properties of the dienophiles, inverse demand Diels-Alder reaction were also investigated using Frontier Molecular Orbital Theory. It is shown that in inverse demand Diels-Alder Reaction, FMOs are the LUMO of diene and HOMO of dienophile. This is because of the changing in electron density in both dienophiles and dienes. Reaction and activation energies were calculated to determine the thermodynamic and kinetic favourable products for different reactions. If the reaction has smaller activation energies, reaction will under kinetic control and forming kinetic product is more favourable. Same principle applies with the thermodynamic controlled reaction, forming thermodynamic product is more favourable if the reaction pathway has greater reaction energies.
Reference
- ↑ Atkins & De Paula Physical Chemistry, 9 edn., 2010.
- ↑ E. M. PopovG. A. KoganV. N. Zheltova, Theoretical and experimental Chemistry,6,pp 11–19
- ↑ Kendall N. Houk, Frontier molecular orbital theory of cycloaddition reactions, Acc. Chem. Res., 1975 8(11), pp.361-369.
- ↑ Zhuang.M et,al Inverse-electron-demand Diels-Alder reactions:principles and Applications,2017, 12(17), pp2142-2159
Appendix
Exercise 1
Exercise 2
- cyclohexadiene(PM6 level): File:RING OP.LOG
- 1,3-Dioxole(PM6 level): File:O op results.log
- Transition State(PM6 level): ENDO(File:ENDO MIN PM6.LOG) EXO(File:EXO BB TS PM6 4.LOG)
Exercise 3
- DA EXO pathway:File:EX3 EXO TS IRC.LOG
- DA ENDO Pathway IRC: File:ENDO IRC 5 correct.LOG
- Cheletropic IRC:File:Lqe ch IRC 2 correct.LOG
Extension
- ENDO Pathway Transition State (PM6):File:Lqe EXT ENDO TSPM6.LOG
- ENDO Pathway Product(PM6):File:EXY ENDO PRODUCT PM6.LOG
- ENDO Pathway IRC: File:EXT ENDO TSPM6 IRC.LOG
- EXO Pathway Transition State (PM6):File:LQE EXT BB PM6.LOG
- EXO Pathway Product(PM6):File:LQE EXT PRODUCT PM6.LOG
- EXOPathway IRC: File:LQE EXT TSPM6 IRC.LOG