Rep:Mod:1008871
Module 1 : Structure and Spectroscopy (Molecular Mechanics and Molecular Orbital)
Molecular Mechanics
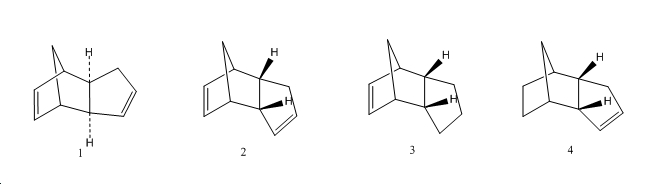
The Hydrogenation of Cyclopentadiene Dimer
The cyclopentadiene dimer 2 hydrogenates to give either product 3 or 4. The energies of all the compounds were minimised and are tabulated below.
| Compound | Stretch kcalmol-1 | Bend kcalmol-1 | Stretch-bend kcalmol-1 | Torsion kcalmol-1 | Non-1,4-VDW kcalmol-1 | 1,4-VDW kcalmol-1 | Dipole/dipole kcalmol-1 | Total Energy kcalmol-1 |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.292 | 20.587 | -0.841 | 7.672 | -1.436 | 4.232 | 0.378 | 31.883 |
| 2 | 1.248 | 20.857 | -0.831 | 9.504 | -1.508 | 4.300 | 0.445 | 34.015 |
| 3 | 1.207 | 18.864 | -0.753 | 12.240 | -1.553 | 5.765 | 0.163 | 35.932 |
| 4 | 1.096 | 14.507 | -0.549 | 12.497 | -1.051 | 4.512 | 0.141 | 31.154 |
From the above values, dimer 1 (exo) has a lower torsion energy and an overall lower energy than dimer 2 (endo). This is backed up by dihedral angle measurements;
| Compound | Dihedral angle |
|---|---|
Dimer 1  |
179.5o |
Dimer 2  |
-46.8o |
Dimer 1 has a dihedral angle closer to 180o which means its conformation is close to that of a staggered conformer and is lower in energy. Dimer 2 has a dihedral angle close to that of an eclipsed conformer which is high in energy. This suggests that dimer 1 would be the easier dimer to form but dimer 2 (kinetic product) is the major product formed as the dimerisation proceeds via the kinetic pathway. This is due to secondary orbital interactions of the HOMO and LUMO which occur only in the formation of the endo dimer.
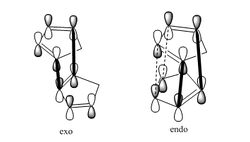
Product 3 has a higher bend energy value and a higher overall energy than product 4 hence is less stable. This is due to the double bond in product 3 being more constrained by the bridge, pushing the angle in the ring to 107o which is less than 120o (sp3 carbon ideally have angles of 120). The double bond in product 4 is less constrained (angle between bonds found to be 115o. Another reason for product 3 being the less stable product is that its double bond is shared between 2 rings while the double bond in product 4 is found in only one ring.
| Compound | Bond Angle |
|---|---|
Product 3  |
107o |
Product 4  |
115o |
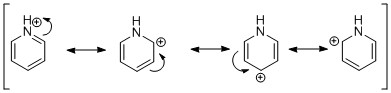
Stereochemistry of Nucleophilic addition to a pyridinium ring (NAD+ analogue)
The MeMgI component was not included in the MM2 optimisation because the program is not parametrised to run for metals. When MM2 was run with the Grignard, an error saying "no atom type was assigned to the correct atom" appeared. The optimisation considers only the mechanics (geometry of the molecule and its transition states) and not the mechanism of the reaction.
Both compounds 5 and 7 were optimised and the dihedral angle between the carbonyl group and the carbon in the C4 position of the pyridinium ring found.
| Compound | Dihedral Angle |
|---|---|
5 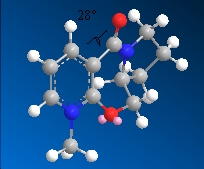 |
28.1o with the carbonyl group pointing out of the plane of the molecule |
7 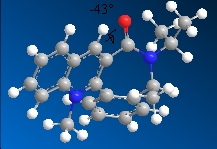 |
-43.1o with the carbonyl group pointing into the plane of the molecule |
The reaction converting molecule 5 to 6 proceeds by chelation of the Grignard to the carbonyl oxygen before the Me group attacks the carbon on the same face as the chelation[2]. In compound 5, the MeMgI chelates to the O on the top face as the carbonyl group is pointing out of the plane of the molecule, therefore the Me attaches onto the pyridine ring on the top face giving the absolute chemistry as shown.
The conversion of molecule 7 to molecule 8 is controlled by sterics[3]. Aniline is a bulky group and since the carbonyl group is pointing into the plane of the molecule, it will attack the ring from the opposite face as the carbonyl oxygen, hence the attack from the top face (resulting in the stereochemistry shown).
Stereochemistry and reactivity of an intermediate in the synthesis of Taxol
The two isomers of the intermediate in the synthesis of Taxol were optimised by MM2 and the energies obtained are tabulated below.
The dihedral angle between the carbonyl oxygen and the neighbouring hydrogen next to the bridge on the same side as the oxygen was also measured:
| Isomer | Dihedral Angle |
|---|---|
10 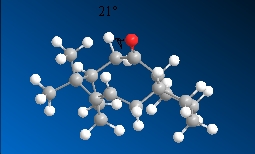 |
21.3o |
11 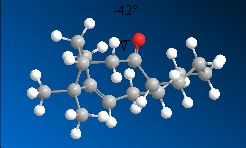 |
-42.5o |
From the above data, isomer 10 has a smaller dihedral angle that is closer to the dihedral angle of an eclipsed conformer which is higher in energy and is hence the more unstable isomer. This is confirmed in the optimised energy obtained for isomer 10 which is higher than the energy for isomer 11.
The alkene in the taxol intermediate reacts slower than normal alkenes because in these isomers, only one end of the double bond can be attacked by electrophiles, the other end is hindered by the bridge. In normal alkenes, there is no hinderance to electrophilic attack at both ends of the double bond hence normal alkenes are more reactive.
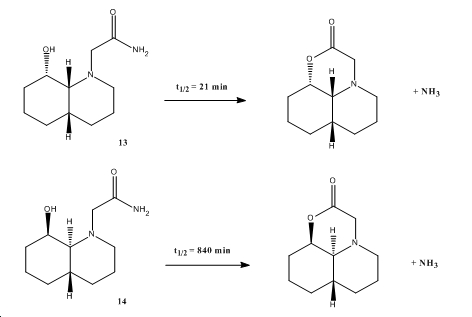
How one might induce room temperature hydrolysis of a peptide
The two isomers of compound 13 and 14 were optimised using MM2 and the position of the OH-group and the ethylamido group in each isomer, and their corresponding minimised energies are tabulated below.
| Isomer | Position of OH-group | Position of ethylamido group | Energy kcalmol-1 | |||
|---|---|---|---|---|---|---|
|
Equatorial | Axial | 19.404 | |||
|
Equatorial | Equatorial | 13.195 | |||
|
Axial | Axial | 12.033 | |||
|
Axial | Equatorial | 9.737 |
From the above energies, it can be seen that the lowest energy isomers have the ethylamido group in the equatorial position. This is because the axial position of the ethylamido group introduces unfavourable 1,3-diaxial interactions with the hydrogens. In all the isomers, hydrogen bonding helps to stabilise the molecules and lower their energy.
Hydrolysis of these compounds occur by intramolecular nucleophilic attack by the hydoxyl group so both the hydroxyl group and the ethylamido group have to be axial or both equatorial[4]. In the hydrolysis of 14, the ethylamido group is equatorial to the axial hydroxyl group and therefore has to invert to the higher energy axial position (both are now axial) which slows down the rate of hydrolysis. This is not required in 13 as both substituents are equatorial so hydrolysis of 13 is much faster.
Both these reactions take less than 500 years to occur because it takes place intramolecularly (both the hydroxyl and ethylamido group are on the same molecule) whereas normal amino acids have each functional group on two separate molecules.
Modelling using Semi-empirical Molecular Orbital Theory
Regioselective Addition of Dichlorocarbene
Compound 12 and its monohydrogenated version was optimised using HF/STO-3G and B3LYP/6-31G(d) methods and their structures shown below:
| Compound 12 HF opt | Compound 12 B3LYP opt | Monohydrogenated 12 HF opt | Monohydrogenated 12 B3LYP opt | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structures |
|
|
|
| ||||||||||||
| C-Cl bond length/Å | 1.797 | 1.789 | 1.799 | 1.792 | ||||||||||||
| C=C bond length/Å | 1.308 | 1.332 | 1.339 | 1.307 |
Both optimisations showed little change in the actual geometry of the molecule. C-Cl and C=C bond lengths taken for each molecule at the different optimisation levels varied only slightly which means both the HF and B3LYP optimisations gave similar optimised structures.
The HF optimisation of compound 12 allowed the molecular orbitals to be illustrated graphically:
| HOMO-1 | HOMO | LUMO | LUMO+1 | LUMO+2 |
|---|---|---|---|---|
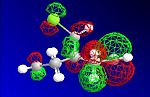 |
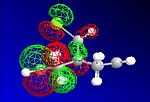 |
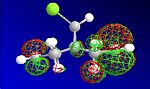 |
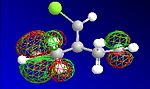 |
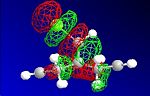
|
From the B3LYP optimisation, the C-Cl and C=C stretching frequencies were obtained.
| C-Cl freq/nm | C=C anti to the C-Cl/nm | C=C freq/nm | |
|---|---|---|---|
| Compound 12 | 772.6 | 1740.8 | 1760.9 |
| Monohydrogenated 12 | 776.9 | - | 1761.7 |
The C-Cl stretching frequency in compound 12 was lower than that for the monohydrogenated version due to C-Cl σ* orbital and the C=C p-orbital interaction which transfers electron density from the σ to σ* orbital, weakening the C-Cl σ-bond that leads to lower stretching frequencies[5]. This orbital interaction is also the reason for the shorter spatial distance between the endo carbon and the bridgehead carbon in compound 12:
| Molecule | Spatial Distance/Å |
|---|---|
Compound 12 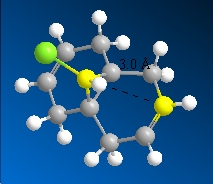 |
3.018Å |
Monohydrogenated 12 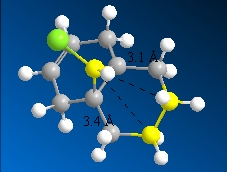 |
3.240Å |
Structure based Mini Project using DFT-based Molecular Orbital methods
Assigning regioisomers in "Click Chemistry"
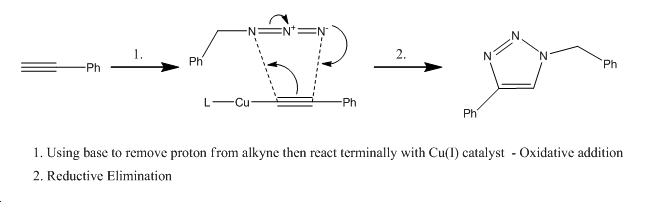
Mechanism of Cu(I) catalysis[6]
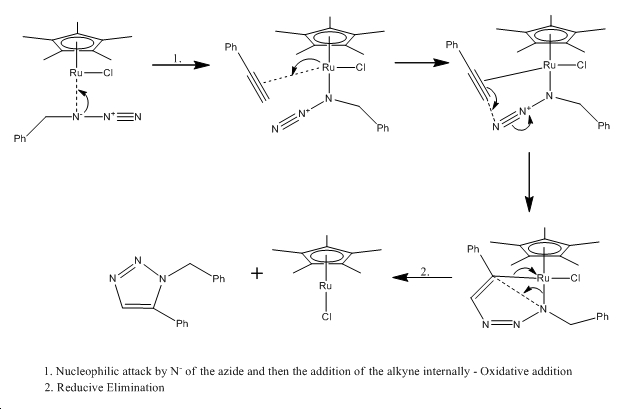
Mechanism of Ru(II) catalysis[7]
The Ru(II) catalyst gives only the 1,5-isomer (isomerB) because the alkyne cannot bind terminally to the catalyst but instead binds internally. The Cu(I) catalyst on the other hand can bind terminally to the catalyst, resulting in the 1,4-isomer (isomerA) being formed almost exclusively.
Isomer A - 1,4 isomer
Isomer B - 1,5 isomer
A key spectroscopic method of differentiating the isomer products in the click reaction is to use 13C NMR spectrometry. Proton NMR spectra obtained by DFT is unreliable and so would not be useful in analysis the isomers. Infrared spectroscopy is also not useful as both products have the same chemical formula and would have similar stretching frequencies. The absence of chiral carbons in the isomers means optical rotation cannot be used to differentiate between them.
The chemical shifts from the 13C NMR spectra obtained by DFT for isomers A and B are tabulated below:
| Isomer A chemical shifts/ppm | 145.1(2) | 133.0(11) | 127.4 | 125.6(15) 125.3(20) 125.1(14) 125.0(13) 124.6(12) 124.4(19) 124.3(16) |
121.9(21) 117.2(3) | 54.7(6) |
|---|---|---|---|---|---|---|
| Literature chemical shifts/ppm [8] | 148.0 | 134.6 | 127.9 | 125.5 | 119.5 | 54.0 |
| Isomer B chemical shifts/ppm | 136.6(3) | 131.7(7) | 130.1(2) | 126.7(18) 126.0(16) 125.5(17) 125.3(15) 124.5(10) |
52.4(6) | |
| Literature chemical shifts/ppm[9] | 138.2 | 135.5 | 133.3 | 126.9 | 51.8 |
Published NMR for 1,4 isomer (A) DOI:10042/to-1173 Published NMR for 1,5 isomer (B) DOI:10042/to-1174
From the above data, the calculated chemical shifts for both isomers matched the literature chemical shifts very well so there was no need to correct for spin-orbit coupling errors. Comparing the chemical shifts of the 1,4-isomer and 1,5-isomer, the highest chemical shift for the 1,4-isomer which corresponded to carbon 2 was larger than that for the 1,5-isomer which corresponded to carbon 3. This means that carbon 2 is more deshielded; the phenyl ring attached to carbon 2 is in the same plane as the triazole which deshields the carbon more. The phenyl ring attached to carbon 3 is in a different plane to the triazole which causes less deshielding of the carbon, hence a lower chemical shift.
References
- ↑ Do Secondary Orbital Interactions Really Exist?, Garcia, J.I., Mayoral, J.A., and Salvatella, L. Acc. Chem. Res., 33, 10, 658 - 664, 2000,DOI:10.1021/ar0000152
- ↑ Regio- and stereoselective control in the addition of Grignard reagents to the pyridine ring system, Arthur G. Schultz, Lawrence Flood, and James P. Springer, J. Org. Chem.; 1986; 51(6) pp 838 - 841, DOI:10.1021/jo00356a016
- ↑ Preparation of axially chiral quinolinium salts related to NAD+ models: new investigations of these biomimetic models as `chiral amide-transferring agents', Stephane Leleu, Cyril Papamicael, Francis Marsais, Georges Dupas, Vincent Levacher, Tetrahedron: Asymmetry Volume 15, Issue 24, 13 December 2004, Pages 3919-3928, DOI:10.1016/j.tetasy.2004.11.004
- ↑ Rapid Cleavage of Unactivated, Unstrained Amide Bonds at Neutral pH, Fernandes, Nicolette M., Fache, Fabienne, Rosen, Mari, Nguyen, Phuong-Lan, and Hansen, David E., J. Org. Chem., 73, 16, 6413 - 6416, 2008, DOI:10.1021/jo800706y
- ↑ A molecular orbital and crystallographic study of the structure and -facial regioselectivity of 9-chloro-1,4,5,8-tetrahydro-4a,8a-methanonaphthalene, Brian Halton, Roland Boese and Henry S. Rzepa, J. Chem. Soc., Perkin Trans. 2, 1992, 447 - 448, DOI:10.1039/P29920000447
- ↑ Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides, Tornoe, C.W., Christensen, C., and Meldal, M., J. Org. Chem., 67, 9, 3057 - 3064, 2002, DOI:10.1021/jo011148j
- ↑ Ruthenium-Catalyzed Cycloaddition of Alkynes and Organic Azides, Zhang, L., Chen, X., Xue, P., Sun, H.H.Y., Williams, I.D., Sharpless, K.B., Fokin, V.V., and Jia, G., J. Am. Chem. Soc., 127, 46, 15998 - 15999, 2005, DOI:10.1021/ja054114s
- ↑ U. Sirion, Y. J. Bae, B. S. Lee and D. Y. Chi, Synlett, 2008, 2326-2330, DOI:10.1055/s-2008-1078245
- ↑ Supporting information for Ruthenium-Catalyzed Cycloaddition of Alkynes and Organic Azides, Zhang, L., Chen, X., Xue, P., Sun, H.H.Y., Williams, I.D., Sharpless, K.B., Fokin, V.V., and Jia, G., J. Am. Chem. Soc., 127, 46, 15998 - 15999, 2005, DOI:10.1021/ja054114s