Rep:Mod:0309rcba
Module 1 - Rachael Bartholomew
Modelling Using Molecular Mechanics
Exercise 1 - The Hydrogenation of a Cyclopentadiene Dimer
Dimerisation of Cyclopentadiene
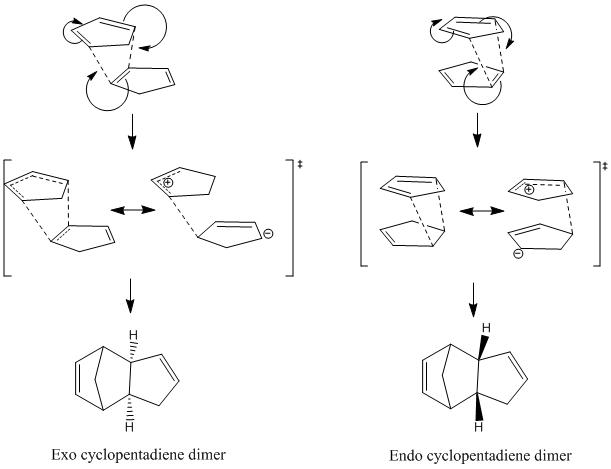
The dimerisation of cyclopentadiene is a Diels-Alder [4+2] cycloaddition reaction. One molecule of cyclopentadiene acts as the 4 π-electron diene, and another acts as the 2 π-electron dienophile. The reaction is stereospecific, where both double bonds are required to be in a cis conformation. This is not an issue when considering cyclic systems, because the double bonds are forced into cis conformations by being in a ring. Two products may be obtained from the reaction, namely the endo and the exo products. In order to study the dimerisation reaction, reactants one and two were drawn, using ChemBio3D Ultra 12.0. After the structures were drawn, their geometries were optimised by using an MM2 calculation to minimise the energy. This provided energy outputs, which were compared to give information about the dimerisation reaction.


| Energies (kcal/mol) | Reactant 1 - exo dimer | Reactant 2 - endo dimer |
| Stretch | 1.3030 | 1.2454 |
| Bend | 20.5869 | 20.8603 |
| Stretch-Bend | -0.8425 | -0.8320 |
| Torsion | 7.6385 | 9.5039 |
| Non-1,4 VdW | -1.4073 | -1.5083 |
| 1,4 VdW | 4.2276 | 4.3012 |
| Dipole/Dipole interactions | 0.3771 | 0.4448 |
| Total energy | 31.8833 | 34.0153 |
This table of data shows that the exo product is more stable overall than the endo product. This can be said because the total energy of the endo dimer is higher in energy than the exo dimer. This difference in energy between the two dimers is due mostly to the increased strain, or torsion energy, arising from increased steric hindrance in the endo product. Predominantly, this reaction produces the endo product, because the formation of the products is under kinetic control. If the reaction is under kinetic control, this means that a product which is less stable is formed as the major product, due to a lower activation energy of this reaction pathway, so the more stable, thermodynamically favoured product is the minor product. In this case, the exo product is the thermodynamic product because it is the more stable of the two. So if the reaction was thermodynamically favoured, the equilibrium between the endo and exo dimer would lie to the side of the exo dimer, due to its lower total energy, and therefore greater thermodynamic stability. The preference for the endo product above the exo product can be explained by considering the transition states for the reaction.
The reaction is kinetically controlled, so the endo transition state must be lower in energy than the exo transition state. By looking at the mechanism above for the dimerisation reaction, it can be seen that the transition state for the endo product is more stable due to the closer proximity of the charges, compared to the exo transition state, where the charges are quite far apart. This means that the endo transition state is more stable, lower in energy, so the preferred product of the kinetically controlled reaction.
This reaction demonstrates that using an MM2 calculation to analyse the reaction has limitations. In this reaction, the transition states of the reaction have to be considered to have a better picture of what is happening, the calculation is not an effective way of analysing a reaction in a kinetic sense. MM2 calculations only take into account the stability of the molecule in terms of the positions of the atoms. In order to study the reaction more thoroughly, the wavefunction of the molecule would need to be taken into account, so the transition states could be modelled.
Hydrogenation of Endo Cyclopentadiene Dimer
Following the dimerisation reaction, the hydrogenation of the endo cyclopentadiene dimer was investigated. The stability of hydrogenating each of the two double bonds was assessed by studying the energies of the two dihydrogenated products. As before, the structures of the two products were drawn in ChemBio3D Ultra 12.0, and their geometries were optimised by using an MM2 calculation to minimise their energies.

| Energies (kcal/mol) | Product 3 | Product 4 |
| Stretch | 1.2794 | 1.1034 |
| Bend | 19.0909 | 14.5151 |
| Stretch-Bend | -0.8432 | -0.5464 |
| Torsion | 11.1365 | 12.5123 |
| Non-1,4 VdW | -1.6454 | -1.047 |
| 1,4 VdW | 5.7864 | 4.4985 |
| Dipole/Dipole interactions | 0.1622 | 0.1408 |
| Total energy | 34.9668 | 31.1767 |
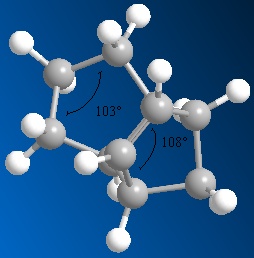
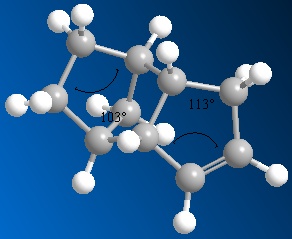
This hydrogenation reaction is thermodynamically controlled. The results show that the dihydrogenation product 4 is overall more stable than product 3. This increase in stability for product 4 could be due to the release of strain due to removing a double bond near to a bridging-group. So, the higher energy of product 3 could be due to this strain, decreasing the stability. By looking at the results, it can be seen that a major contribution to the overall energy is made by the bending components of the energy. The bending energy shows the extent of the deviation from the ideal bond angle. This energy is higher in product 3 than in product 4. The diagrams below show the key bond angles in products 3 and 4.
| Product 3 | Product 4 |
|---|---|
 |
|
It can be seen from these diagrams of bond angle calculation, that the double bond in product 3 is more strained. This can be said because the angle shown across the double bond is 108o. Ideally, this angle would be 120o, due to the sp2 hybridised carbon atoms involved in the bonding. In the case of product 4, the angle shown is 113o, which is much closer to the ideal value. This is seen in the energy results because the bending energy of product 4 is lower than product 3 due to a smaller deviation from the ideal bond angle across the double bond. By hydrogenating the most strained double bond in the endo product 2, near the carbon bridging-group, to yield product 4, there is a greater stabilisation, leading to a greater decrease in the total energy of the product. This decrease in energy is the driving force for the thermodynamically controlled reaction, therefore product 4 is the major product of the hydrogenation reaction.
Exercise 2 - Stereochemistry of Nucleophilic Additions to a pyridinium ring (NAD+ analogue)
This exercise involves the investigation of two prolinol derivatives, and the nucleophilic addition to their pyridine rings. The energies of the two structures were minimised, and the geometry of the carbonyl group was related to the reaction mechanisms of the two examples.
Alkylation of pyridine ring of prolinol derivative using MeMgI
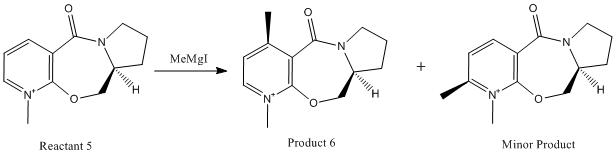
The addition reaction between reactant 5 and MeMgI could produce two products. One is product 6, following alkylation of the pyridine ring in the 4-position by the grignard reagent MeMgI. The other, minor product would have the pyridine ring alkylated in the 2-position.

In order to investigate why this preference for product 6 might exist, the structure of reactant 5 was drawn in ChemBio3D Ultra 12.0. The geometry was optimised by using an MM2 calculation, to minimise the energy. The results are shown in the table below.
| Energy (kcal/mol) | Reactant 5 |
|---|---|
| Stretch | 2.0309 |
| Bend | 14.1623 |
| Stretch-Bend | 0.1315 |
| Torsion | 5.2200 |
| Non-1,4 VdW | -0.6036 |
| 1,4 VdW | 16.5350 |
| Charge/Dipole Interactions | 9.6628 |
| Dipole/Dipole Interactions | -3.9817 |
| Total Energy | 43.1573 |
These results were obtained after running several MM2 calculations from different starting conformations of the molecule. The positions of the atoms were moved before the geometry of the molecule was optimised. This trial and error approach showed that the end result of an MM2 calculation depends strongly on the initial conformation of the molecule. So the optimised structure obtained after the calculation may not, in fact, be the lowest energy conformation of the molecule, only by moving the atoms around manually into more accurate positions may the lowest energy conformation be achieved. The lowest energy conformations were found to have the carbonyl group slightly above the plane of the aromatic ring in the molecule. When the carbonyl group was moved below the plane, the total energy of the molecule rose significantly to 124.91 kcal/mol, clearly an unstable conformation. The angle between the aromatic ring and the carbonyl is shown in the diagram below.
 |
|
|---|---|
| Diagram to show dihedral angle in reactant 5 between carbonyl group and the plane of the aromatic ring |
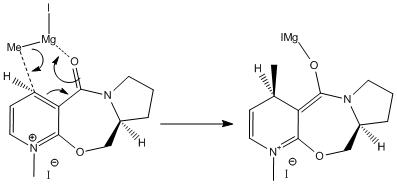
The angle between the carbonyl group and the plane of the aromatic ring was found to be 12.1o. This geometry of the carbonyl group in more stable conformations of reactant 5 can be related to the reason for the preference for product 6 in the addition reaction with grignard reagent, and the resulting stereochemistry of the methyl group added to the pyridine ring.
The carbonyl group was found to lie slightly above the plane of the aromatic ring in the molecule, so observing the key steps in the mechanism in the diagram above, it can be seen that because the electrophilic magnesium coordinates to the carbonyl group, the methyl group will add to the molecule on the same face as the carbonyl group, above the plane of the pyridine ring. This chelating effect is why product 6 is favoured over the product alkylated in the 2-position of the pyridine ring.
When investigating this model of reactant 5, it was seen that when the grignard reagent was included in the model, the optimisation of the geometry failed, and an error message was returned. This is because the MM2 method works by using set parameters, from known, defined molecules. For some types of bonding, functional groups and atoms, parameters are not defined. Parameters for metal atoms are not available using this method, so the magnesium included in the grignard reagent cannot be handled by this method.
Reaction of pyridinium ring of Quinolinium salt using Aniline
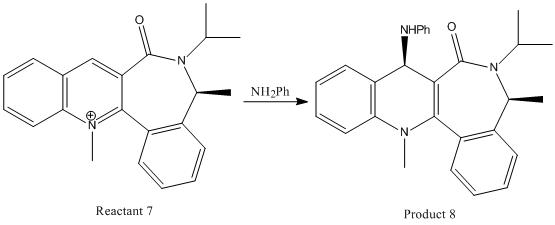
The investigation of this reaction also involves studying the stereochemistry of the product, and how this might have been affected by the carbonyl group in the molecule. The reaction to be studied is shown below. The reaction shows the quinolinium salt, reactant 7, is attacked by the nucleophile aniline (NH2Ph) to obtain product 8, where the NHPh group has been added to the pyridinium ring. The NHPh group is positioned above the plane of the molecule in product 8. The reason for this can be explained by studying reactant 7, the quinolinium salt.
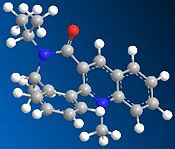
Reactant 7 was drawn in ChemBio3D Ultra 12.0, and the geometry was minimised by an MM2 calculation to minimise the energy of the molecule. The table below shows these results
| Energies (kcal/mol) | Reactant 7 |
|---|---|
| Stretch | 3.7001 |
| Bend | 11.773 |
| Stretch-Bend | 0.3989 |
| Torsion | 9.8595 |
| Non-1,4 VdW | 4.1128 |
| 1,4 VdW | 29.3232 |
| Charge/Dipole Interactions | 1.9536 |
| Dipole/Dipole Interactions | -4.875 |
| Total Energy | 56.2460 |
The results are useful to see that the lowest energy conformation, and therefore the most stable, has been achieved. This conformation of the molecule will then be used to analyse the geometry of the carbonyl group, in order to relate this to the mechanism of the nucleophilic addition reaction. In order to achieve these results for the lowest energy conformation, many trial and error minimisations were completed, some with quite high energies, for example 98.512kcal/mol. This high energy indicated that there was still too much strain in the structure. In order to minimise the energy of the molecule further, the positions of the atoms in the 7- and 5-membered rings of the structure were moved manually to try and obtain a more reasonable result. Again, this exercise shows that the inital positions of the atoms in the molecule affect the end results of MM2 calculations, and it is important to try and accurately position the atoms in order to achieve a lower energy conformation of the molecule. Having minimised the energy to 56.246kcal/mol as shown above, the dihedral angle between the plane of the aromatic pyridinium ring and the carbonyl group was calculated.
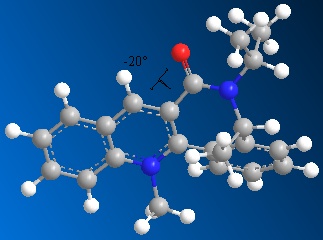 |
|
|---|---|
| Diagram to show the dihedral angle between the carbonyl group and the plane of the aromatic rings |
This diagram shows that the carbonyl group actually lies slightly below the plane of the molecule, at -20o. This result can be used when considering the mechanism of the reaction, and the resulting stereochemistry shown in product 8. The aniline in this mechanism, unlike the grignard reagent seen before, will not coordinate to the carbonyl group due to repulsive coulombic interactions between the two. These repulsive interactions arise due to the high electron density in both the double bond of the carbonyl group and the phenyl ring of the aniline molecule; and lone pair repulsion between the oxygen of the carbonyl group and the nitrogen atom in the aniline molecule. The aniline will therefore add to the pyridinium ring from the least hindered face of the molecule, to minimise steric repulsion. This face will be the opposite face to the carbonyl group, above the plane of the pyridinium ring, leading to the NHPh group in product 8 positioned above the plane of the molecule.
As noted for both these nucleophilic reactions, using the MM2 calculation has limitations, due to the result of the calculation depending on the initial guess of the geometry of the molecule. The MM2 method also could not handle the magnesium atom in the calculation either, without returning an error message. In order to study these reactions in more detail, MOPAC/PM6 methods could be used in order to take into account the molecular wavefunction. This method is used below.
Exercise 3 - Stereochemistry and Reactivity of an Intermediate in the Synthesis of Taxol
Stereochemistry of Intermediates
This exercise involves two intermediates in the synthesis of Taxol, the two molecules are atropisomers. Atropisomerism arises from restricted rotation about single bonds, where the barrier to rotation is due to steric strain[3]. In the case of these two molecules, the isomerism arises from the rotation of the carbonyl group, and the rigid structure of the substituted rings in the molecule are too hindered to allow free rotation. This results in two isomers of the intermediate, molecule 9 where the carbonyl group faces upwards, and molecule 10, where the carbonyl group faces downwards. In the synthesis of these intermediates, both isomers will be formed, but when left to stand, rotation of the carbonyl group will take place, leaving only the most stable isomer present.

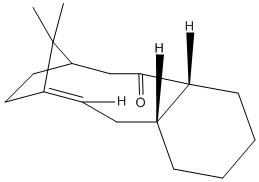
Both molecules 9 and 10 were drawn in ChemBio3D Ultra 12.0, and their geometries were optimised using an MM2 calculation. The results are shown below.
These results show that the most thermodynamically stable isomer is molecule 10, where the carbonyl group is pointing down. This can be said because the total energy of the molecule is lower than for molecule 9. So after a mixture of isomers was left to stand, after a period of time it would be expected that all the molecules of intermediate would have the conformation of molecule 10, as this is more stable.
The lowest energy conformations of both molecules have the six-membered cyclohexane ring arranged in a chair conformation. When molecule 9 is initially minimised, the six membered ring is in a twist-boat conformation, resulting in a higher total energy of the molecule equal to 54.431kcal/mol. This energy was further minimised by manually moving the atoms into the more stable chair conformation and running the MM2 calculation again. Mainly the torsion and bending energies are changed when changing from twist-boat to chair, this reduction in these energies is due to the relief of strain in the molecule, and less deviation from the ideal bond angles.
The difference in energy of the two isomers can be explained when the stereoelectronic effects are considered.
| Molecule 9 | Molecule 10 |
|---|---|
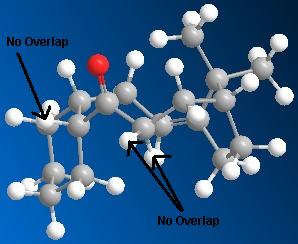 |
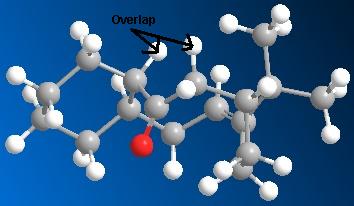
|
These diagrams show that in molecule 10 the two hydrogen atoms indicated are antiperiplanar to the carbonyl group, this allows orbital overlap between the π*-C=O orbital and the σ-C-H orbitals of the adjacent hydrogens. This hyperconjugation interaction stabilises the molecule, lowering the total energy. This stabilisation does not occur in molecule 9. The overlap of the orbitals is not possible due to the relative geometry of the carbonyl and adjacent hydrogens, they are not anti-periplanar. Therefore molecule 10 is more stable, and lower in energy than molecule 9.
Reactivity of Alkene Bond in Intermediates
It was noted that after the formation of these intermediates, it was difficult to hydrogenate the alkene bond in the molecule. This suggests that the product alkane is less stable than the alkene. More commonly, the alkane is more stable, lower in energy than the corresponding alkene. This is due to a positive Olefin Strain Energy (OSE)[4]. On the other hand, some cycloalkenes show negative OSE, showing that the alkene is, in fact more stable than the alkane. This greater stability of the alkene is due to increased strain in the alkane. More specifically, increased torsion and van der Waals energies result from hydrogenation due to small dihedral angles of the new C-H bonds and repulsion between hydrogen atoms which are positioned too close together, within the van der Waals radius of each other. So, the slow hydrogenation reaction of the alkene bond in the intermediate could be due to the alkene having a negative OSE. This would make the alkane thermodynamically unfavourable, compared to the alkene, so it would be expected that the hydrogenation reaction would be slow.
Modelling Using Semi-Empirical Molecular Orbital Theory
Exercise 4 - Regioselective Addition of Dichlorocarbene
Orbital Control of Reactivity
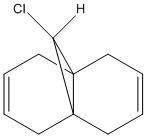
This exercise involves considering the reactivity of the two alkene bonds in molecule 12 by investigating the molecular orbitals. It is noted from the literature that reaction with an electrophile is regiospecific, the alkene bond endo to the chlorine will react. The aim of this exercise is to explain this selectivity by studying the molecular orbitals of the molecule, and relating these results to the reactivity of the molecule.
In order to investigate the molecular orbitals, molecule 12 was drawn in ChemBio3D Ultra 12.0, and the geometry was optimised using an MM2 calculation to minimise the energy. The total energy of the molecule was found to be 17.897kcal/mol after this calculation. The main component of this energy is from the torsional strain 7.633kcal/mol, due to the rigid ring structure of the molecule.
Following the MM2 minimisation, a MOPAC PM6 calculation was used in order to generate graphic representations of the molecular orbitals. After the MOPAC minimisation, the energy of the molecule rose to 19.740kcal/mol.
| Method | Total Energy (kcal/mol) | |
|---|---|---|
| MM2 | 17.897 |
|
| MOPAC PM6 | 19.740 |
|
Following the MOPAC PM6 minimisation, the molecular orbitals could be shown for the molecule. These results are shown below. The orbitals are shown as translucent in order to see clearly where they lie on the molecule.
| HOMO-1 | 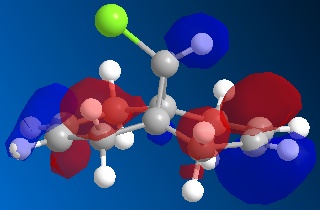
|
| HOMO | 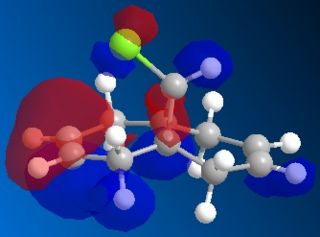
|
| LUMO | 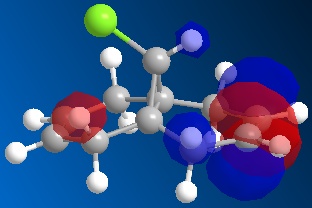
|
| LUMO+1 | 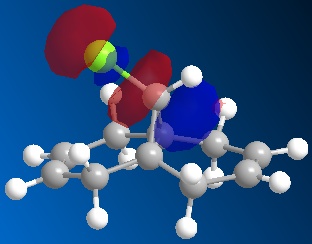
|
| LUMO+2 | 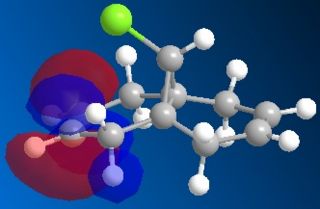
|
These results can explain the reactivity of the alkene bond endo to the chlorine in the molecule 12. By looking at the diagram for the HOMO (Highest Occupied Molecular Orbital) it can be seen that there is a lot of electron density around the endo alkene bond. This electron density gives rise to the observed reactivity with electrophiles. The increased reactivity of the endo alkene bond could also be considered as an indication that the exo alkene bond to the chlorine could be stabilised by favourable π-C=C to σ*-C-Cl interactions[5]. This overlap helps to stabilise the exo alkene bond, and results in a more nucleophilic endo alkene bond.
Influence of C-Cl Bond on Vibrational Frequencies

In order to investigate the influence of the C-Cl bond on the vibrational frequencies of the molecule, two molecules will be studied: molecule 12, and a dihydrogenated product-molecule 12a, where the exo alkene bond has been hydrogenated.
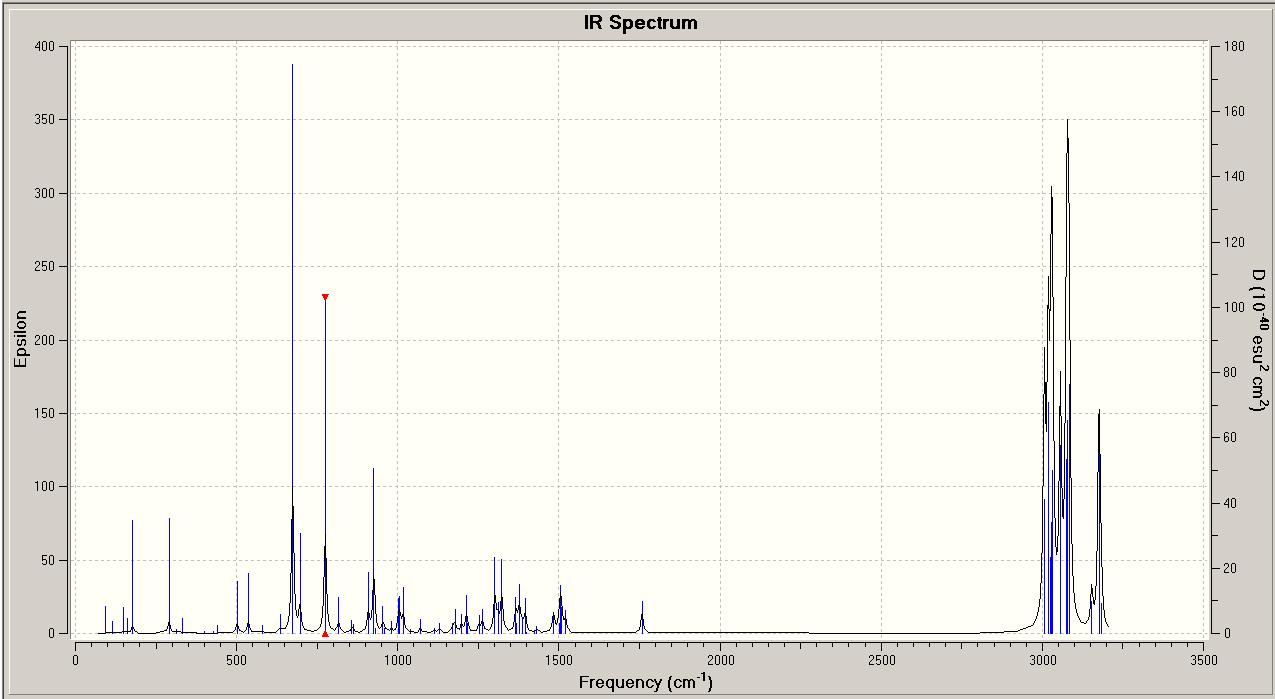
In order to study the stretching frequencies of the molecules, they were drawn in ChemBio3D Ultra 12.0, and minimised using an MM2 calculation, followed by a MOPAC PM6 optimisation. The optimised molecules were saved as Gaussian Input Files, and sent to a more powerful computer to complete the calculations. The output files have been used to draw predicted Infra-Red spectra of the molecules, and the alkene bond and carbon-chlorine bond stretches associated with these vibrational frequencies, using GaussView.
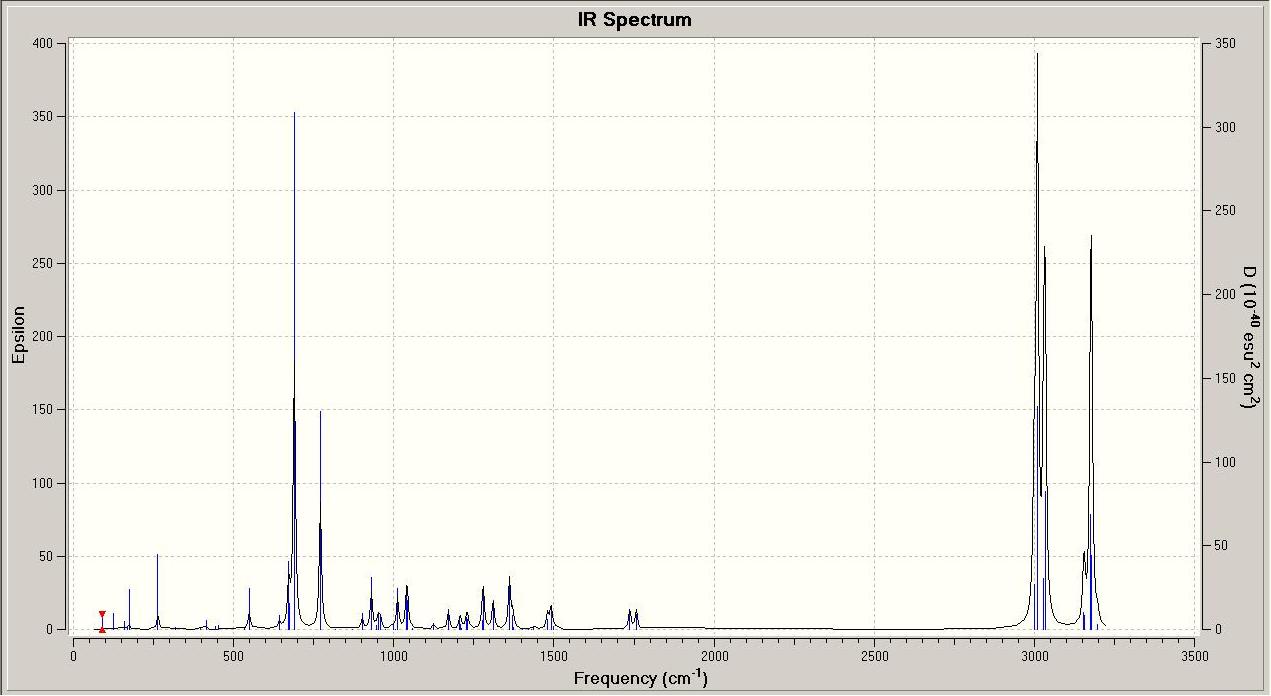
|
|---|
| Stretch | Diagram | Frequency of Vibration (cm -1) |
|---|---|---|
| Exo C=C bond | 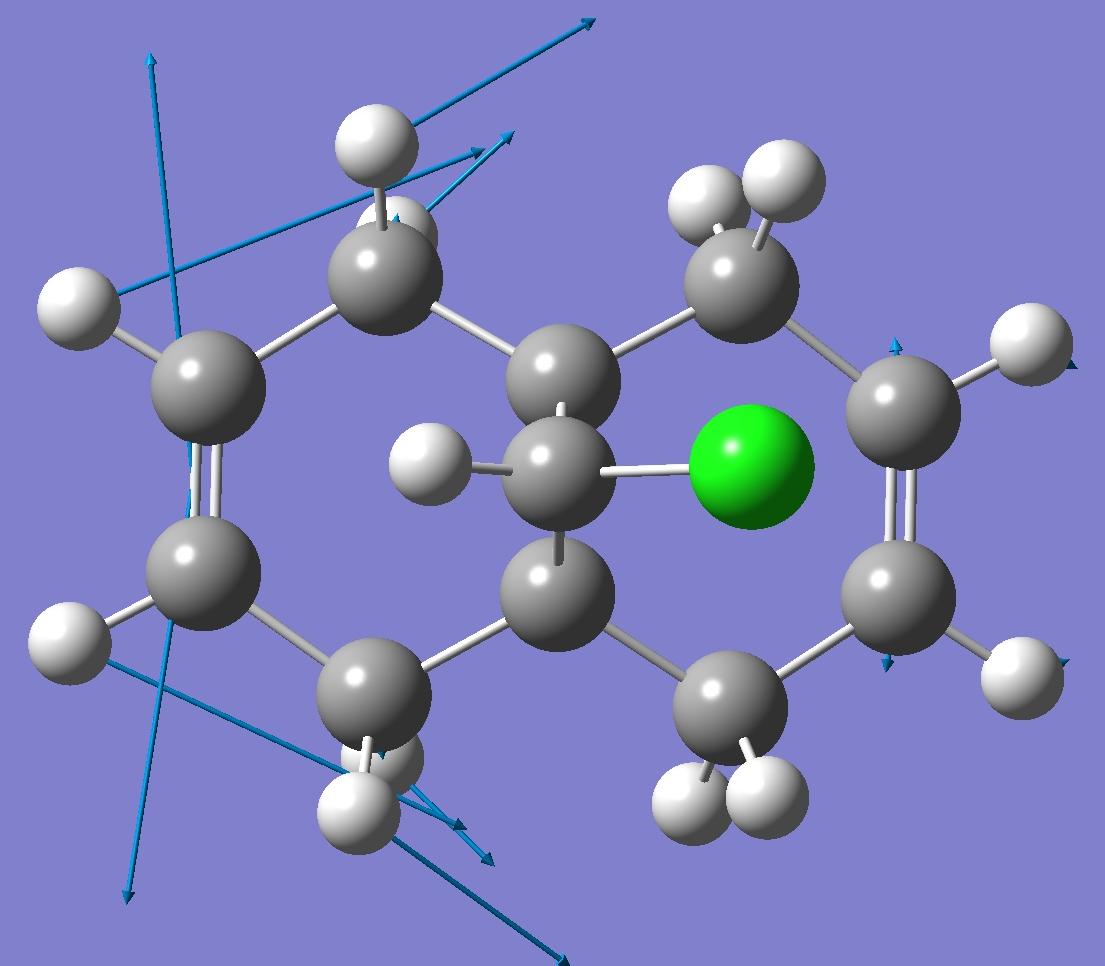 |
1737.02 |
| Endo C=C bond | 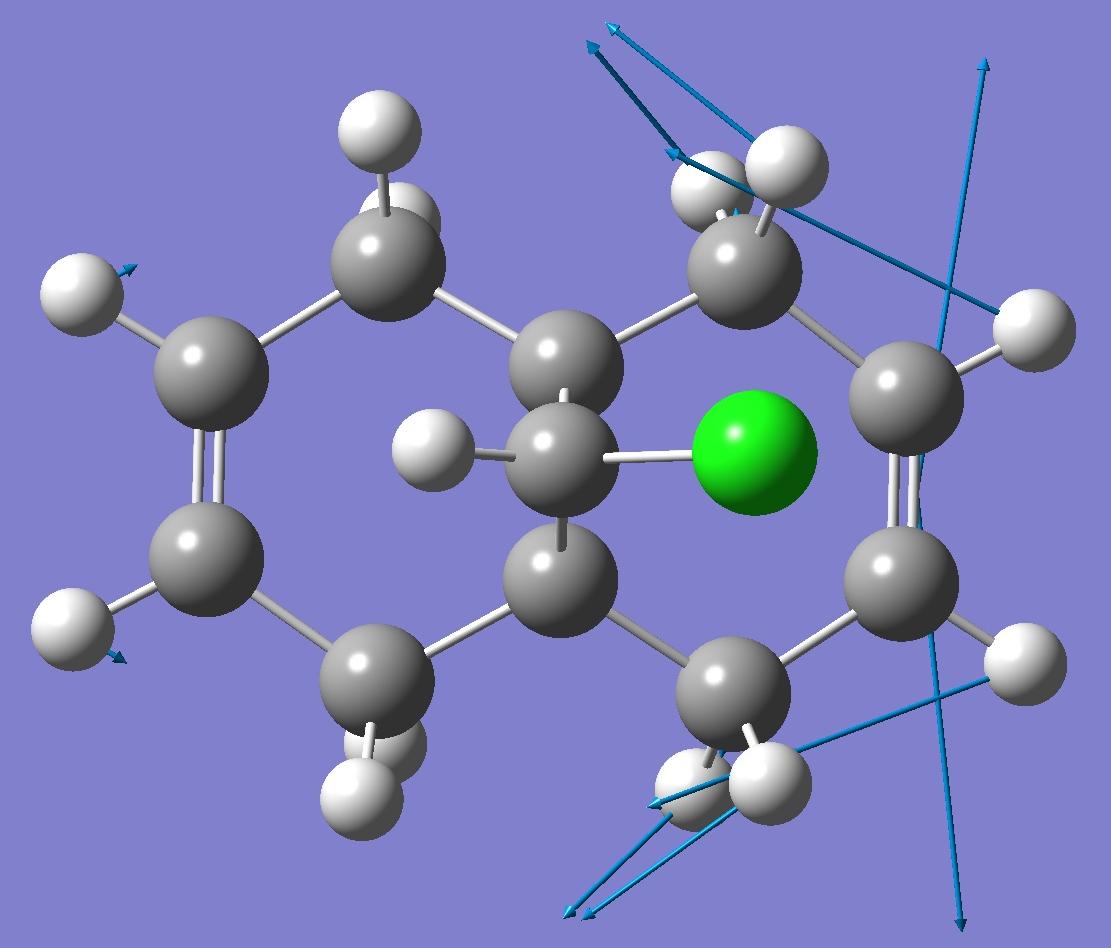 |
1757.34 |
| C-Cl bond | 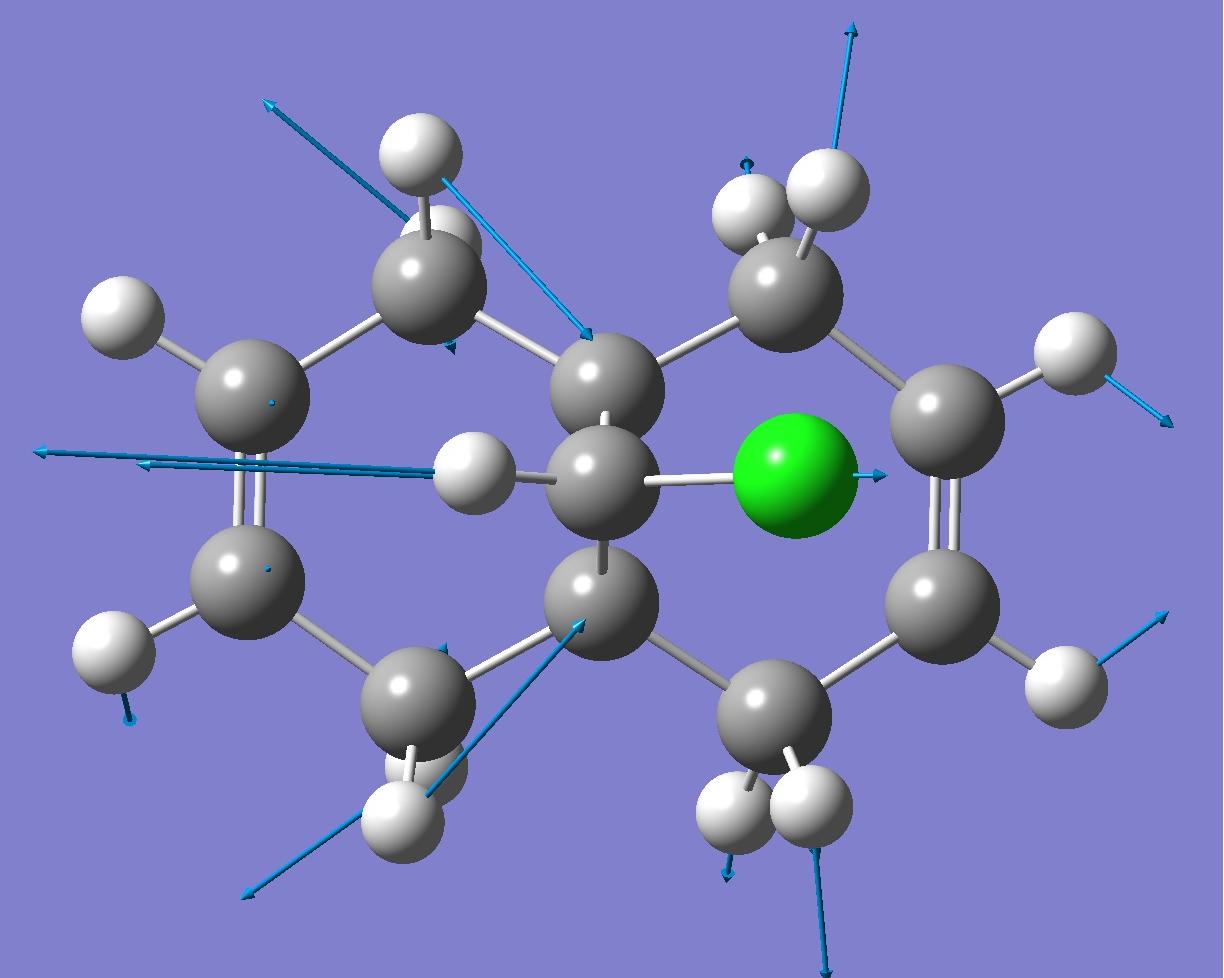 |
770.88 |
|
|---|
| Stretch | Diagram | Frequency of Vibration (cm -1) |
|---|---|---|
| Exo C-C bond | 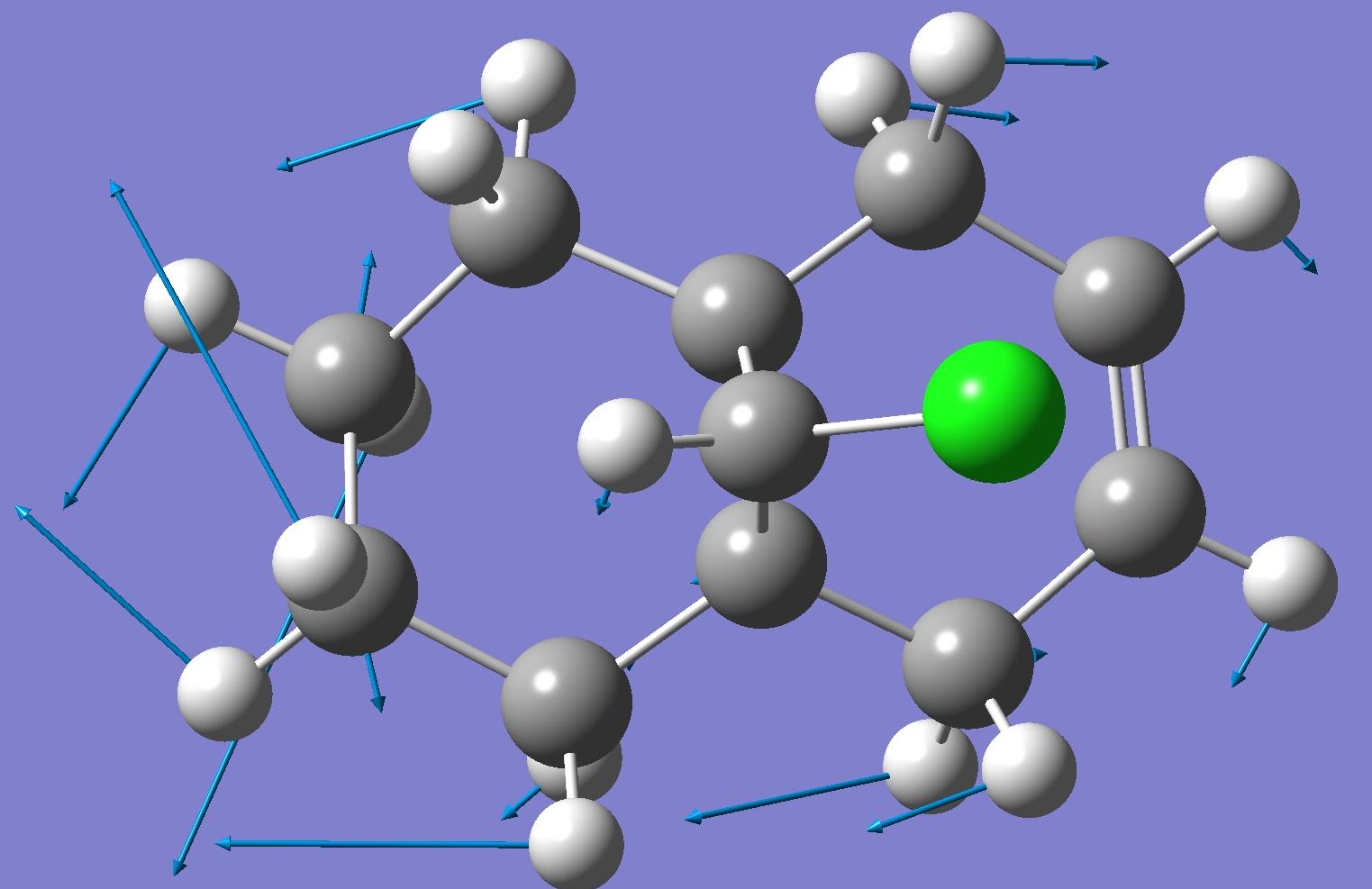 |
1395.63 |
| Endo C=C bond | 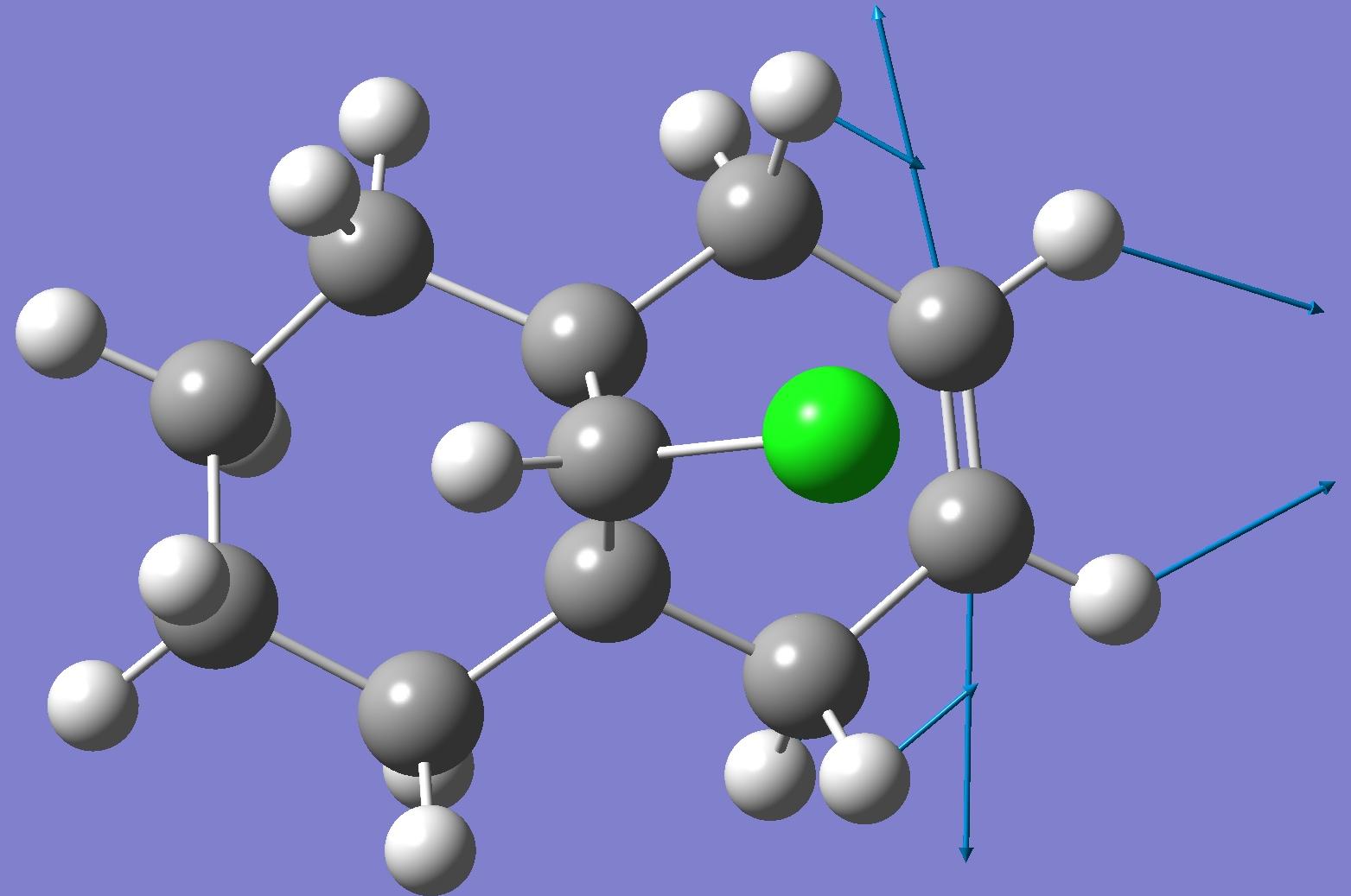 |
1758.06 |
| C-Cl bond |  |
774.96 |
By looking at these results it can be seen that the C-Cl bond stretching frequency increases for the monoalkene, 12a compared to the dialkene. This can be explained by considering the molecular orbital investigation above. The dialkene molecule is stabilised by a π-C=C(exo C=C bond) and σ*-C-Cl orbital overlap, however, this interaction weakens the C-Cl bond due to increased electron density in an anti-bonding orbital. The weaker C-Cl bond has a lower vibrational frequency (770.88cm -1). In the monoalkene the vibrational frequency of the C-Cl bond (774.96cm -1) is higher. This increase in wavenumbers is due to a stronger C-Cl bond, the exo-alkene has been hydrogenated, so there is no longer a π-σ* interaction.
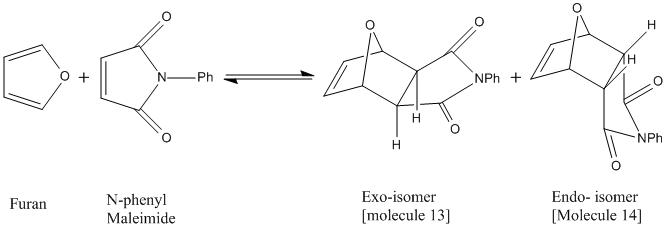
Mini-Project: Endo and Exo Selectivity in the Diels-Alder Reaction of N-Phenyl Maleimide with Furan
This project involves the reaction between N-Phenyl maleimide with furan to give either endo- or exo-7-oxabicyclo[2.2.1]hept-5-ene-2,3-dicarboxy-N-phenylimide. The reaction is found to give a mixture of isomers. At ambient temperatures, or higher, the exo isomer is the major product, but at lower temperatures (0oC), the endo isomer is the major product. This reaction therefore exhibits typical behaviour where the endo product is the kinetic product and the exo isomer is the thermodynamic product. The aim of this project is to show why this thermodynamic vs. kinetic control may be, and how isomers may be identified by using 13C NMR, 1HNMR or Infra-Red spectra. These predicted spectra, obtained from computational methods could then be used to show how the different isomers could be identified from their spectra if they were produced in the lab. The predicted spectra generated will be compared to known, literature values, and assessed as to whether differences between the two isomers are distinct. The reaction scheme is shown below.
The endo and exo isomers were drawn in ChemBio3D Ultra 12.0, and minimised using an MM2 calculation, followed by a MOPAC PM6 calculation. The results are shown below.
These results show that the endo isomer has a higher total energy compared to the exo isomer. This agrees with the observed preference for each isomer at different temperatures from the literature[6]. The exo isomer is the more stable isomer, with a total energy 1.6321kcal/mol lower than the endo isomer. This is not a big difference in energy, but the ratio of products at ambient temperature is 68% exo isomer; 23% endo isomer and 9% starting material (N-phenyl maleimide). This equilibrium of products was reached after 20days. After 7 days, under the same conditions the mixture of products was: 41% endo isomer, 48% exo isomer and 11% starting material. These observed results show that the exo isomer is the more stable, thermodynamic product of the reaction, and that when the reaction mixture is left for a longer length of time, due to equilibration, more of the exo isomer can form. The endo isomer is the kinetic product, this is seen by the lower total energy, and observed in the literature results, when the temperature was decreased, the endo isomer was the major product, even though the endo isomer has a higher total energy, the energy of the transition state may be lower than for the exo isomer, so at lower temperatures, the reaction to form the endo isomer is faster.
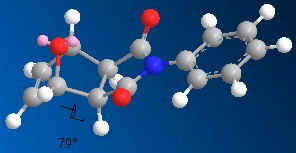
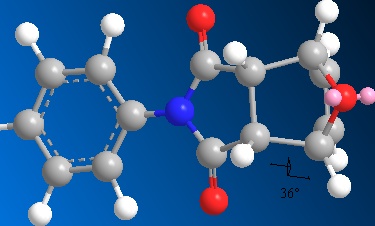
One contribution to the difference in energy between the two isomers can be explained by looking at key dihedral angles in the molecules.
| Exo Isomer | Endo Isomer |
|---|---|
 |
|
These dihedral angles show why the endo isomer has a larger bending energy compared to the exo isomer, as the dihedral angle deviates further from the ideal case. The literature values [6] for these angles are 83.0o (exo) and 33.8o (endo). These differ slightly from the values obtained here, but the literature values were achieved using a MOPAC AM1 calculation, which could account for the difference.
Another contribution to the difference in the energy of the two isomers is due to steric factors. There is less steric hindrance if the 3-atom bridge eclipses the anhydride ring (in the exo isomer) than if the 4-atom bridge is eclipsing the anhydride ring (in the endo isomer)[7]. This difference means that the exo product is more stable than the endo isomer.
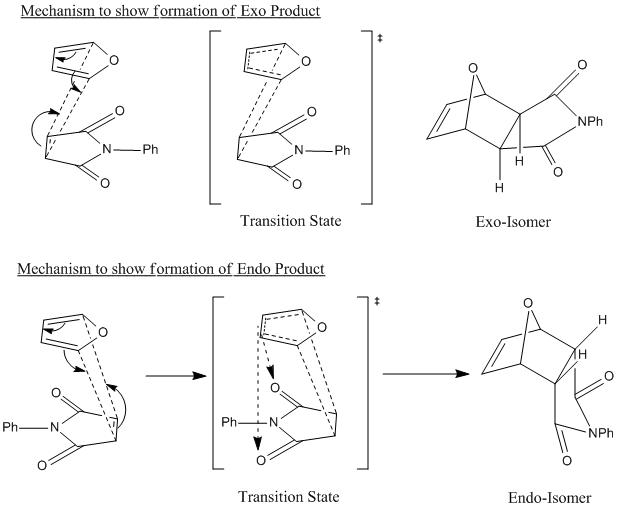
By thinking about the mechanism for the reaction it can be seen why the endo product is the kinetically favoured product. The transition state of the endo isomer must be lower in energy than the transition state to form the exo isomer, leading to a lower activation barrier to the formation of the endo product.
This mechanism shows how the endo isomer is expected to have a lower energy transition state. This is due to bonding interactions between the forming alkene bond in the furan ring, and the carbonyl groups of the anhydride ring. These interactions are shown with dotted arrows. This bonding interaction stabilises the transition state of the endo product, relative to the exo product, as these interactions are not possible in the transition state of the exo product due to the arrangement of the reactants. Therefore the endo isomer will form more quickly than the exo isomer.
Predicting 13CNMR spectra for Endo and Exo Products and Comparison to Literature
In order to investigate whether the two isomers can be separated spectroscopically, the minimised conformations were used to predict the 13CNMR spectra of the two isomers. These predicted spectra have been compared to the literature in order to see if the isomers could be separated in a lab by their NMR spectra.
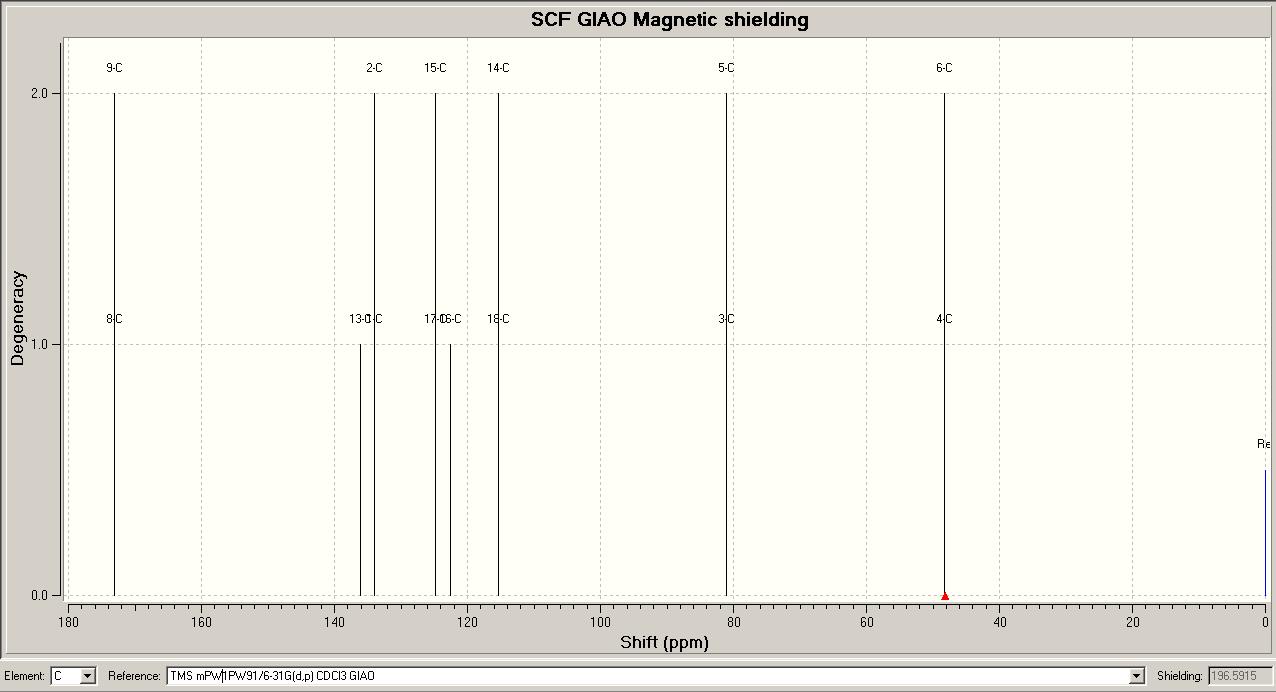
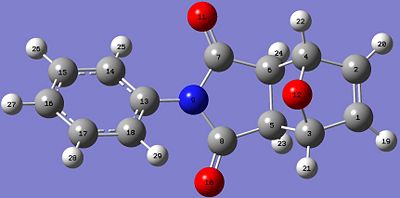
Predicted 13CNMR spectrum for Endo Isomer DOI:10042/to-4316
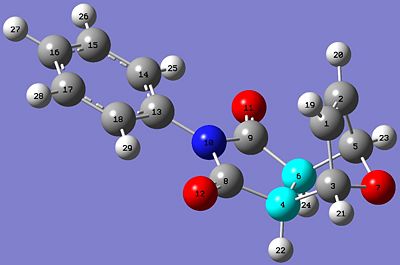
Predicted 13CNMR spectrum for Exo Isomer DOI:10042/to-4317
| Exo Isomer | Endo Isomer |
|---|---|
 |
|
| Endo isomer | Exo Isomer | ||||
| Literature values[6] | Predicted values | Literature values[6] | Predicted values | ||
| Chemical shift (δ/ppm) | Carbon atom label | Chemical shift (δ/ppm) | Chemical shift (δ/ppm) | Carbon atom label | Chemical shift (δ/ppm) |
| 173.9 | 8, 9 | 173.1 | 175.3 | 8, 7 | 171.7, 171.4 |
| 134.6 | 13 | 136.0 | 136.7 | 2, 1 | 136.5, 136.3 |
| 131.4 | 1, 2 | 134.0 | 131.5 | 13 | 129.7 |
| 129.2 | 15, 17 | 124.8 | 129.1 | 17, 15 | 124.9, 124.8 |
| 128.8 | 16 | 122.6 | 128.8 | 16 | 123.8 |
| 126.3 | 14, 18 | 115.4 | 126.5 | 14, 18 | 121.8, 121.6 |
| 79.8 | 3, 5 | 81.0 | 81.4 | 3, 4 | 82.4, 82.3 |
| 45.9 | 4, 6 | 48.2 | 47.5 | 6, 5 | 50.2, 50.1 |
These results from the predicted 13CNMR are mostly in good agreement with the literature values. The least similar values compared to the literature for both isomers are the carbon atoms in the aromatic phenyl ring: C14,15,16,17,and 18 for both isomers. The chemical shifts for these carbon atoms are quite different from the literature values. This could be due to the molecules having different conformations. The aromatic ring is reasonably flexible in this structure, and this method of predicting the spectra is very sensitive to conformational variation in the structures. However, all other chemical shifts are quite close to the literature values.
The results do show that the 13CNMR spectra of the two isomers are different, but only by a very small margin, so this type of NMR could not be used to definitely identify each isomer.
Predicting 1HNMR spectra for Endo and Exo Products and Comparison to Literature
In addition to the 13CNMR spectra, the 1HNMR spectra can also be predicted for the two isomers. The experimental spectra of both isomers are included in the literature, so the predicted spectra will be compared to experimental results.
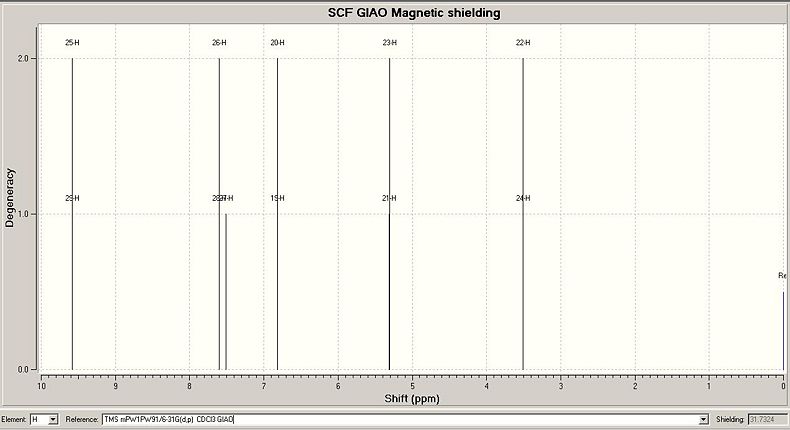
Predicted 1HNMR Spectrum for Endo Isomer
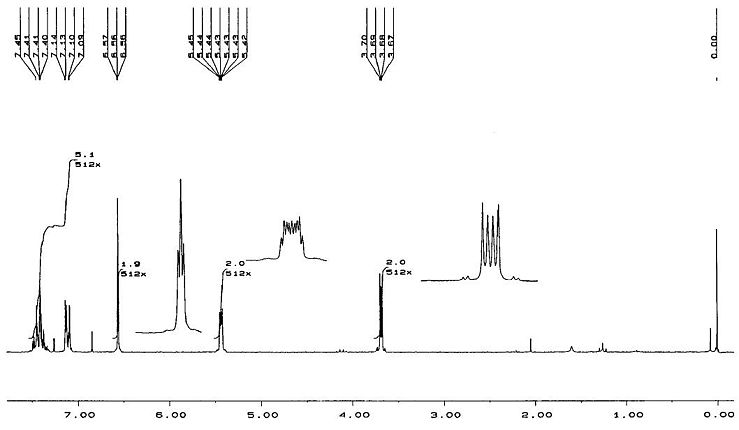
Literature[6] 1HNMR Spectrum for Endo Isomer
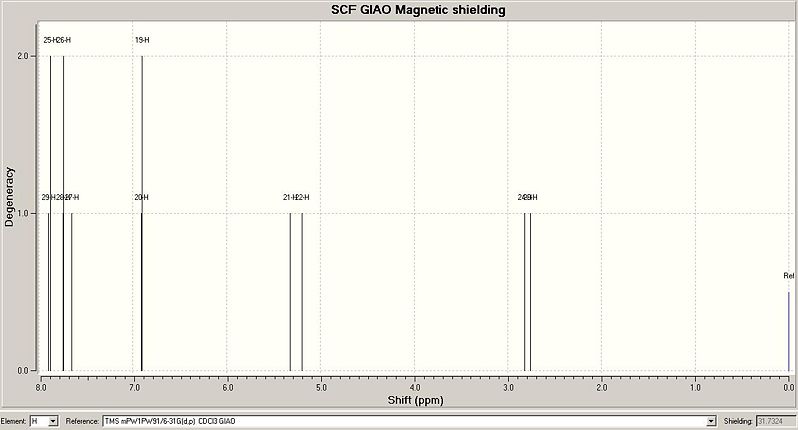
Predicted 1HNMR Spectrum for Exo Isomer
Literature[6] 1HNMR Spectrum for Exo Isomer
| Endo isomer | Exo Isomer | ||||
| Literature[6] values | Predicted values | Literature[6] values | Predicted values | ||
| Chemical shift (δ/ppm) | Hydrogen atom | Chemical shift (δ/ppm) | Chemical shift (δ/ppm) | Hydrogen atom | Chemical shift (δ/ppm) |
| 7.43 | 25, 29 | 9.58 | 7.45 | 29 | 7.90 |
| 7.12 | 26, 28 | 7.60 | 7.45 | 28, 25 | 7.74, 7.88 |
| 7.12 | 27 | 7.51 | 7.30 | 27, 26 | 7.65, 7.74 |
| 6.57 | 20, 19 | 6.82 | 6.56 | 19, 20 | 6.90, 6.91 |
| 5.43 | 23, 21 | 5.30 | 5.38 | 22, 21 | 5.19, 5.32 |
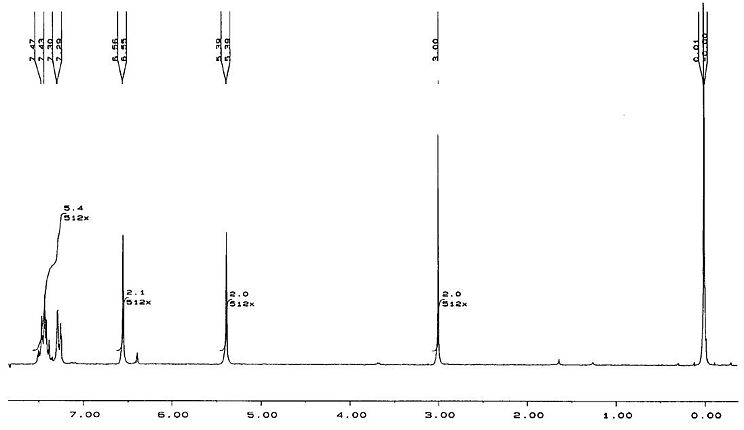
| 3.69 | 22, 24 | 3.51 | 3.00 | 23, 24 | 2.76, 2.82 |
These results from the predicted 1HNMR spectra use the same labels for atoms shown in the section for 13CNMR. The results which are shown in italics (for aromatic protons) in the table are shown in the literature as multiplets with 5H integration. The other peaks in the predicted spectra can be compared directly to peaks which appear in the literature spectra, as shown in the results table. All proton shifts other than the aromatic protons have chemical shifts in good agreement with the literature values. The predicted spectra do not show mutiplets well, so it is difficult to directly compare the literature to the predicted spectra for the aromatic protons. However, for the exo isomer, the chemical shifts of the aromatic protons match the literature values for the multiplet more closely than for the endo isomer. For the endo isomer, hydrogens 26,27 and 28 have reasonable chemical shift predictions, but hydrogens 25 and 29 have a very high predicted chemical shift of 9.58. These hydrogen atoms are bonded to carbon atoms 14 and 18, which also had poor predicted chemical shifts in the 13CNMR spectrum of the endo isomer. These errors in the predicted values could be due to the conformation of the molecule used for predicting the spectra, as discussed above.
The predicted 1HNMR spectra do show differences between the two isomers. In particular the chemical shifts for H24 and H23(exo), and for H22, H24(endo). These chemical shifts are far enough apart (difference=0.69ppm(lit) 0.72ppm(predicted)) to be able to distinguish between the two isomers. The difference may not seem that large, but on a 0-10ppm scale, this difference would be quite easy to see. The difference between other chemical shift values for endo and exo isomers may not be this large, but even a small difference would show noticeably on an 1HNMR spectrum. Therefore this spectroscopic method could potentially be used to differentiate between the two isomers.
Predicting IR spectra for Endo and Exo Products and Comparison to Literature
In addition to the 13CNMR spectrum, an infra-red spectrum could be used to differentiate between the two product isomers. By predicting the two IR spectra of the isomers, it can be seen whether it is possible to tell spectroscopically which isomer is which. In order to obtain the predicted IR spectra for the two isomers, an MM2 optimisation, followed by a MOPAC AM1 calculation, to minimise the energy of the molecule. These conformations of the molecules were then saved as gaussian input files, and sent to a more powerful computer. Gaussview was used to predict the IR spectra from the results.
Predicted IR Spectrum for Endo Product
| Stretch | Diagram | Frequency (cm-1) |
|---|---|---|
| Alkene C=C |  |
1652.76 |
| Asymmetric C=O | 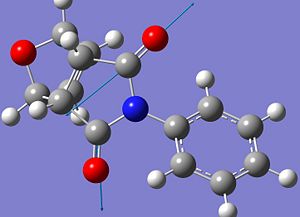 |
1803.59 |
| Symmetric C=O | 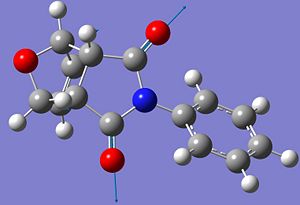 |
1859.85 |
| C-N bond | 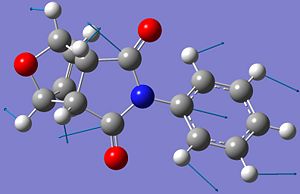 |
1385.42 |
Predicted IR Spectrum for Exo Product
| Stretch | Diagram | Frequency (cm-1) |
|---|---|---|
| Alkene C=C | 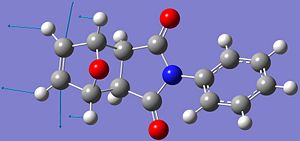 |
1651.63 |
| Asymmetric C=O |  |
1804.26 |
| Symmetric C=O | 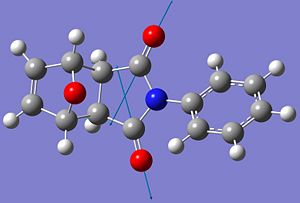 |
1861.58 |
| C-N bond | 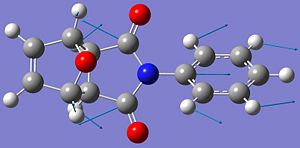 |
1392.56 |
| Product | Frequency (cm-1) |
|---|---|
| Endo | 1713, 1696 |
| Exo | 1712 |
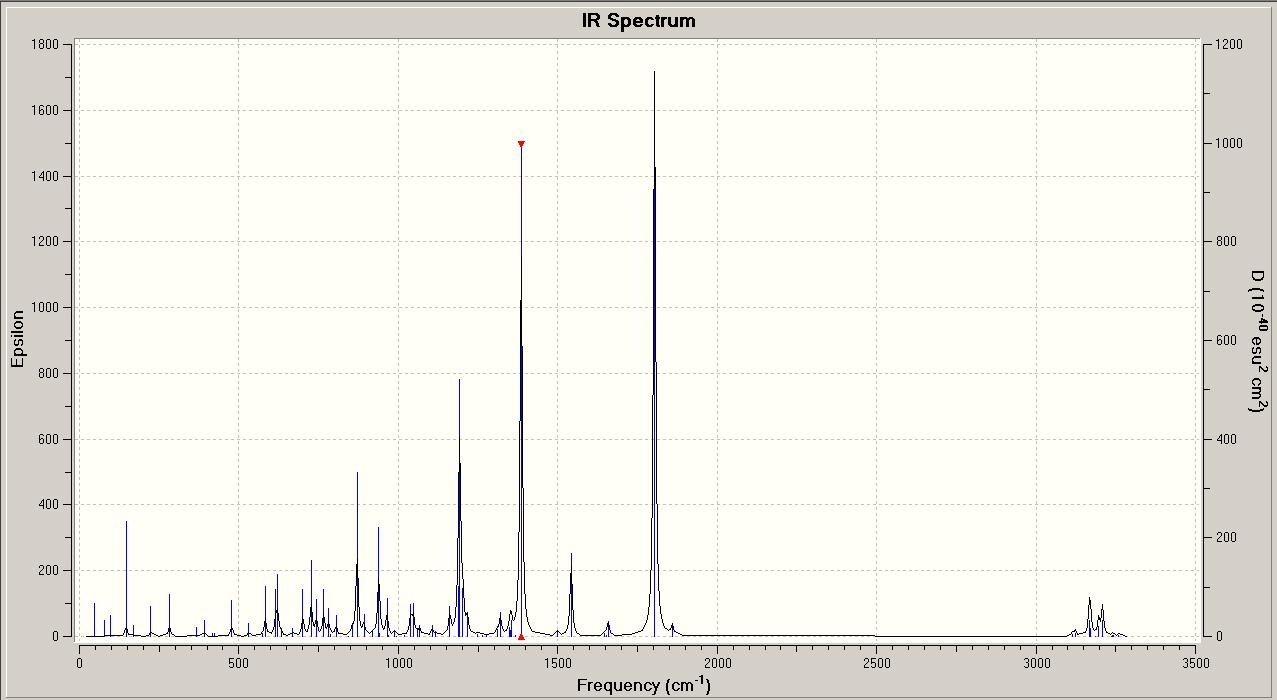
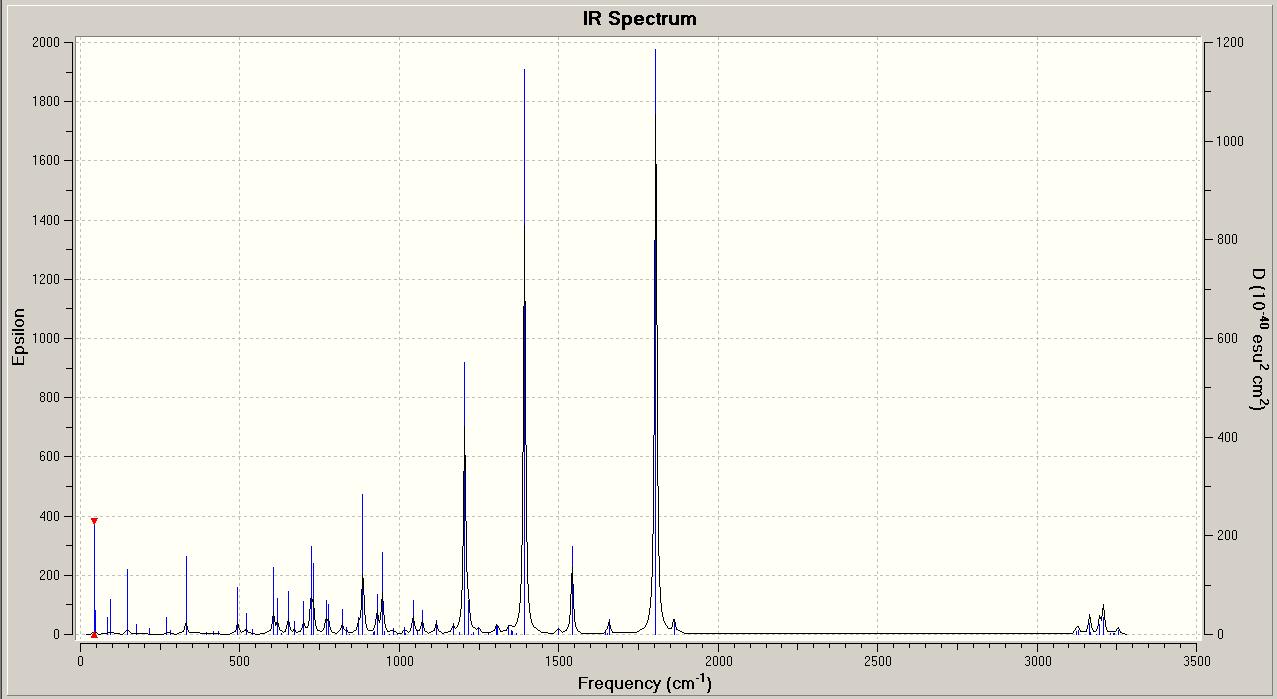
These results from the predicted IR spectra are quite poor. In comparison to the literature values for the carbonyl stretches, the predicted C=O stretching frequencies are too high by almost 100cm-1. However, there are known errors for stretching frequencies in this method of predicting the IR spectra of molecules, so this large difference could be due to this. Also, it is difficult to compare fully the predicted IR spectra to reported values, as only the carbonyl stretching frequencies are listed.
However, these results are useful in that they show that there are differences between the two IR spectra for the isomers. In particular the C-N stretching frequencies are quite different for the isomers, 1385.42cm-1 (endo) and 1392.56cm-1 (exo). If the isomers had been synthesised in the lab, it would be possible to identify either isomer by the molecule's C-N stretching frequency. The other vibrations shown are different between the isomers, but still too close to be able to use the vibrational frequencies to definitely tell the isomers apart.
Conclusion to Mini Project
It has been shown for the reaction between N-Phenyl maleimide with furan to give either endo- or exo-7-oxabicyclo[2.2.1]hept-5-ene-2,3-dicarboxy-N-phenylimide, that under room temperature or higher temperatures the reaction shows thermodynamic control, and the major product is found to be the exo isomer. This is because the exo product is lower in energy than the endo isomer. If the reaction is carried out at lower temperatures, the kinetic endo product is formed due to the transition state for this product being stabilised by a favourable bonding interaction between the carbonyl groups of the anhydride ring and the forming π-bond. This interaction lowers the energy of the transition state, lowering the energy barrier to the formation of the endo product.
By optimising the geometry of the two isomer structures, the 13CNMR, 1HNMR and IR spectra for both isomers were obtained. These spectra were compared with literature values for the expected spectra. It was found that in general 13CNMR and 1HNMR agreed with literature values, but the IR spectra did not due to errors in the values of the predicted vibrational frequencies for the stretches of the molecule. If the isomers were made in the lab 1HNMR spectra could be used to differentiate between the two isomers, but the other methods shown here, 13CNMR and IR do not show enough difference between the isomers to tell them apart.
References
- ↑ Diels-Alder Reaction http://www.ch.ic.ac.uk/motm/porphyrins/introDA.html
- ↑ 2.0 2.1 Regio- and Stereoselective Control in the Addition of Grignard Reagents to the Pyridine Ring SystemDOI:10.1021/jo00356a016
- ↑ Atropisomerism http://en.wikipedia.org/wiki/Atropisomerism
- ↑ Cross-coupling of a functionalized highly pyramidalized alkene: DSC and NMR study of the [2+2] retrocycloaddition of cyclobutane cross products, hyperstability and pyramidalization of the formed dienes, Camps, P.; Pujol, X.; Vazquez, S.; Pericas, M.; Puigjaner, C.; Sola, L., Tetrahedron, 2001, 57, 8511-8520DOI:10.1016/S0040-4020(01)00802-X
- ↑ A molecular orbital and crystallographic study of the structure and π-facial regioselectivity of 9-chloro-1,4,5,8-tetrahydro-4a,8a-methanonaphthalene, B. Halton, R. Boese and H. S. Rzepa., J. Chem. Soc., Perkin Trans 2, 1992, 447. DOI:10.1039/P29920000447
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 Endo- and Exo-Stereochemistry in the Diels–Alder Reaction:
Kinetic versus Thermodynamic Control, J. H. Cooley and R. Vaughan Williams, Journal of Chemical Education, Vol. 74, No. 5, 1997, 582-585 DOI:10.1021/ed074p582 Cite error: Invalid
<ref>tag; name "ed074p582" defined multiple times with different content - ↑ 7.0 7.1 J. Clayden, N. Greeves, S. Warren, P. Wothers, Organic Chemistry, Oxford University Press, 2006, Pages 907-913