Rep:Mod:0307rcba
Exercise 1 - The Hydrogenation of a Cyclopentadiene Dimer
Dimerisation of Cyclopentadiene
In order to study the dimerisation reaction, reactants one and two were drawn, using ChemBio3D Ultra 12.0. After the structures were drawn, their geometries were optimised by using an MM2 calculation to minimise the energy. This provided energy outputs, which were compared to give information about the dimerisation reaction.


| Reactant 1 - exo dimer | Reactant 2 - endo dimer | |
| Stretch | 1.3030 | 1.2454 |
| Bend | 20.5869 | 20.8603 |
| Stretch-Bend | -0.8425 | -0.8320 |
| Torsion | 7.6385 | 9.5039 |
| Non-1,4 VdW | -1.4073 | -1.5083 |
| 1,4 VdW | 4.2276 | 4.3012 |
| Dipole/Dipole interactions | 0.3771 | 0.4448 |
| Total energy | 31.8833 | 34.0153 |
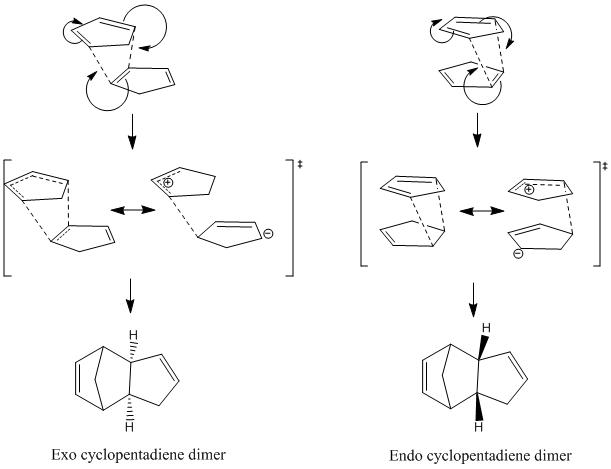
The dimerisation of cyclopentadiene is a Diels-Alder [4+2] cycloaddition reaction. One molecule of cyclopentadiene acts as the diene, and another acts as the dienophile. Predominantly, Diels-Alder reactions produce the endo product, when the formation of the products is under kinetic control. If the reaction is under kinetic control, this means that a product which is less stable is formed as the major product, due to a lower activation energy of this reaction pathway, so the more stable, thermodynamically favoured product is the minor product. This table of data shows that the exo product is overall more stable than the endo product. This can be said because the total energy of the endo dimer is higher in energy than the exo dimer. This difference in energy of the endo dimer is due mostly to the increased strain, or torsion energy. This suggests that the kinetically controlled dimerisation reaction produces the endo product as the major product. If it were thermodynamic, the equilibrium between the endo and exo dimer would lie to the side of the exo dimer, due to its lower total energy, and therefore greater thermodynamic stability. The preference for the endo product above the exo product can be explained by considering the transition states for the reaction.
 <ref name="http://www.ch.ic.ac.uk/motm/porphyrins/introDA.html">
<ref name="http://www.ch.ic.ac.uk/motm/porphyrins/introDA.html">
The reaction is kinetically controlled, so the endo transition state must be lower in energy than the exo transition state. By looking at the mechanism for the dimerisation reaaction, it can be seen that the transition state for the endo product is more stable due to the closer proximity of the charges, compared to the exo transition state, where the charges are quite far apart. This means that the endo transition state is more stable, lower in energy, so the prefered product of the kinetically controlled reaction. Hydrogenation of Endo Cyclopentadiene Dimer
Following the dimerisation reaction, the hydrogenation of the endo cyclopentadiene dimer was investigated. The stability of hydrogenating each of the two double bonds was assessed by studying the energies of the two dihydrogenated products. As before, the structures of the two products were drawn in ChemBio3D Ultra 12.0, and their geometries were optimised by using an MM2 calculation to minimise their energies.


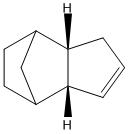
| Product 3 | Product 4 | |
| Stretch | 1.2794 | 1.1034 |
| Bend | 19.0909 | 14.5151 |
| Stretch-Bend | -0.8432 | -0.5464 |
| Torsion | 11.1365 | 12.5123 |
| Non-1,4 VdW | -1.6454 | -1.047 |
| 1,4 VdW | 5.7864 | 4.4985 |
| Dipole/Dipole interactions | 0.1622 | 0.1408 |
| Total energy | 34.9668 | 31.1767 |
The results show that the dihydrogenation product 4 is overall more stable than product 3. This increase in stability for product 4 could be due to the release of strain due to removing a double bond near to bridge-head carbons. So, the higher energy of product 3 could be due to this strain, decreasing the stability. By looking at the results, it can be seen that a major contribution to the overall energy is made by the bending components of the energy. This energy is higher in product 3 than in product 4. By comparison of the bending energies of these dihydrogenation products to the bending energies of the cyclopentadiene dimers, the bending energy of product 4 is much lower than the dimers(reactants 1 and 2), and product 3. The bending energy of product 3 is much more similar to the energies of the reactants 1 and 2. From studying the energies of the two dihydrogenation products it can be said that the more thermodynamically favoured product is 4.
