Rep:Mod:01546737
Jacob Blackmore IMM2 report
NH3
Calculation method: RB3LYP
Basis set: 6-31G(d.p)
Final energy: -56.5577687 au
RMS gradient: 0.0000049 au
Point group: C3V
N-H bond distance: 1.02Å
H-N-H bond angle: 37.1°
NH3 |
The optimisation file for NH3 can be found here
Checking the optimization of NH3
Item Value Threshold Converged?
Maximum Force 0.000004 0.000450 YES
RMS Force 0.000004 0.000300 YES
Maximum Displacement 0.000072 0.001800 YES
RMS Displacement 0.000035 0.001200 YES
Predicted change in Energy=-5.986298D-10
Optimization completed.
-- Stationary point found.
This table is taken from the file produced by the optimisation process. It shows that the force and displacement of the atoms present in the molecule have converged about a stable equilibria, meaning that the molecule has successfully been optimised.
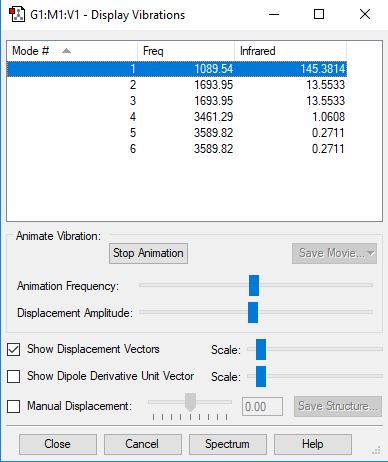
Frequency and charge analysis for NH3
Mode Frequency Infrared 1 1090 145 2 1694 14 3 1694 14 4 3461 1 5 3590 0 6 3590 0
| Wavenumber (cm-1) | symmetry | inensity (arbitrary units) |
|---|---|---|
| 1090 | A1 | 145 |
| 1694 | E | 13.6 |
| 1694 | E | 13.6 |
| 3461 | A1 | 1.06 |
| 3590 | E | 0.271 |
| 3590 | E | 0.271 |
- Using the 3N-6 rule, it would be expected that 6 vibrational moades would be present, where N is the number of atoms present in the molecule.
- The modes 2 and 3, and 5 and 6 are degenerate respectively as they have the same frequency and IR values.
- The bending modes are 1, 2 and 3, whilst the stretching modes are 4,5 and 6.
- Mode 4 is highly symmetric.
- Mode 1 is known as the umbrella mode.
- You would expect to see 4 bands in the experimental spectrum of gaseous ammonia.
- The relative charge on each of the H atoms in NH3 is 0.375, whilst the relative charge of the N atom is -1.125.
N2
Calculation method: RB3LYP
Basis set: 6-31(d.p)
Final energy: -109.5235911 au
RMS gradient: 0.0247309 au
Point group: Dinf
N≡N bond distance: 1.11Å
N≡N bond angle: 180°
N2 |
The optimisation file for N2 can be found here
Checking the optimisation of N2
Item Value Threshold Converged?
Maximum Force 0.000001 0.000450 YES
RMS Force 0.000001 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000000 0.001200 YES
Predicted change in Energy=-3.400981D-13
Optimization completed.
-- Stationary point found.
See 'Checking the opimisation of NH3' for an explanation of this table.
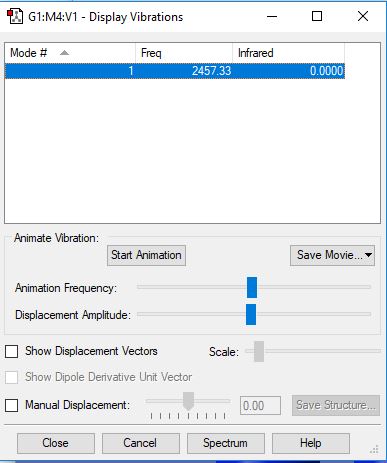
Frequency and charge analysis for N2
Mode Frequency Infrared 1 2457 0.0000
| Wavenumber (cm-1) | symmetry | inensity (arbitrary units) |
|---|---|---|
| 2457 | SGG | 0.00 |
The relative charge on each of the N atoms is 0.00, as they are identical and neither one is more electronegative than the other, meaning that there is no dipole moment present. This results in the value of 0.000 in the infrared spectra, as there must be a permanent dipole moment present for the the vibration to be present in the spectra.
H2
Calculation method: RB3LYP
Basis set: 6-31G(d.p)
Final energy: -1.1785394 au
RMS gradient: 0.0000002 au
point group: Dinf
H-H bond distance: 0.743Å
H-H bond angle: 180°
H2 |
The optimisation file for H2 can be found here
Checking optimisation of H2
Item Value Threshold Converged?
Maximum Force 0.000000 0.000450 YES
RMS Force 0.000000 0.000300 YES
Maximum Displacement 0.000000 0.001800 YES
RMS Displacement 0.000001 0.001200 YES
Predicted change in Energy=-1.164080D-13
Optimization completed.
-- Stationary point found.
See 'Checking the opimisation of NH3' for an explanation of this table.
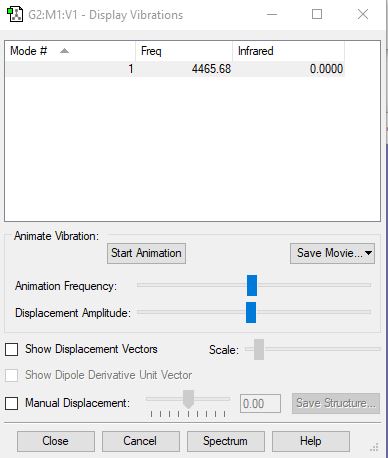
Frequency and charge analysis for H2
Mode Frequency Infrared 1 4466 0.0000
| Wavenumber (cm-1) | symmetry | inensity (arbitrary units) |
|---|---|---|
| 4466 | SGG | 0.00 |
See the 'Frequency and charge analysis for N2' for an explanation for no infrared value present. The relative charge on each of the H atoms is 0.000
Comparison to a mono-metallic transition metal complex
The transition metal complex found coordinates H2. It is called Chloro-dihydrogen-tetrakis(methyldiphenylphosphine)-rhenium tetrahydrofuran solvate, and has the unique refcode GIKGOW01[[1]]. The bond length of H-H in this transition metal complex was found to be 0.908Å, in comparison to the 0.743Å bond length of the H-H molecule. One of the reasons for this difference in bond length may be because the bond lengths found using Gaussian were found through an optimisation process, whilst those reported in Mercury were recorded from experimental results. Alternatively, the bond length of H-H in the transition metal complex may be greater than that of H2 as there may be reduced electron density in the bond due to a donation of an electron pair to the central rhenium atom to form the ligand. This in turn would mean that the force holding the atoms in place would be lessened, increasing the bond length.
Determining the energy for the reaction N2 +3H2 -> 2NH3
E(NH3)= -56.5577687 au
2*E(N3)= -113.1155375 au
E(N2)= -109.5235911 au
E(H2)= -1.1785394 au
3*E((H2)= -3.5356180 au
ΔE= 2*E(NH3)-[E(N2)+3*E(H2)]= -0.0563283 au
ΔE= -147.9 KJmol-1
The ammonia product is more stable than the gaseous reactants
An investigation into SH2
Calculation method: RB3LYP
Basis set: 6-31(d.p)
Final energy: -399.3916241 au
RMS gradient: 0.0001207 au
Point group: C2v
S-H bond distance: 1.35Å
H-S-H bond angle: 92.7°
SH2 |
The optimisation file for SH2 can be found here
Checking the optimisation of SH2
Item Value Threshold Converged?
Maximum Force 0.000175 0.000450 YES
RMS Force 0.000145 0.000300 YES
Maximum Displacement 0.000386 0.001800 YES
RMS Displacement 0.000386 0.001200 YES
Predicted change in Energy=-1.208487D-07
Optimization completed.
-- Stationary point found.
See 'Checking the opimisation of NH3' for an explanation of this table.
Frequency and charge analysis for SH2
Mode Frequency Infrared 1 1224 4.9220 2 2692 6.7347 3 2712 8.6219
| Wavenumber (cm-1) | symmetry | inensity (arbitrary units) |
|---|---|---|
| 1224 | A1 | 4.92 |
| 2692 | A1 | 6.73 |
| 2712 | B2 | 8.62 |
The relative charge on the Sulphur atom is -0.313, whilst the relative charge on each of the Hydrogen atoms is 0.156.
Analysing the Molecular Orbitals of SH2





This molecular orbital is the 2s orbital of S, and the electrons involved are too close to the core, or deep in energy to be able to interact with the 1s electons of hydrogen, and therefore is not involved in bonding. The 2s orbital has an energy of relatively large magnitude, with a value of -7.95115 au.
The molecular orbital shown here is a 2p orbital of the S atom. Again, the electrons are too deep in energy to be able to interact with the 1s electrons in each of the hydrogen atoms. It's energy is also relatively large in magnitude (although not as great as the 2s orbital), and has a value of -5.91588 au. This MO is not involved in bonding.
An in-phase overlap between the 3s orbital of the s atom and the 1s orbital of hydrogen are shown in this molecular orbital. As an in-phase overlap is occuring, this is a bonding orbital, which is filled. This overlap is able to occur as the 3s orbital is shallow enough in energy to interact with the 1s orbitals of each of the H atoms. This molecular orbital is again relatively lower in energy (although there is a significant drop from each of the 2p energies), and has an energy of -0.74654. This MO is needed for the formation of each of the sigma bonds in the SH2 molecule. This has the highest bonding character of the MOs as it is the deepest bonding orbital in energy.
The out-phase overlap between a 3p orbital of S and the 1s orbital of H is shown in this MO. The 3p orbital is shallow in energy in comparison to 2p and 2s. The MO has an energy of -0.44963. As an in phase interaction is present, this MO is a bonding orbital. In addition this MO is occupied. As this is a bonding orbital and is filled, the force constant of the bonds is increased whilst the bond lengths are decreased.
This final MO shows the out of phase interaction between the 1s orbitals of the H atoms and a 3p orbital of S. In addition to an out of phase interaction is occuring, this is an anti-bonding orbital. As an anti-bonding orbital is present, it is not unexpected that a positive value for the energy is reported. This value is 0.02126 au. As with the last three MOs, the 3p orbital shallow enough in energy to allow for interaction with the 1s orbitals of each of the H atoms. This MO is in fact the LUMO, and is therefore unoccupied. As this MO is the LUMO, it is very important for the chemical reactions which SH2 are involved with, and one of the lobes (pointing away from the molecule) must be filled for one of the S-H bonds to be broken. 4 lobes are present as the spins do not overlap.
SbF5 (independent work)
Calculation method: RB3LYP
Basis set: 6-31G(d.p)
Final energy: -504.7207291 au
RMS gradient: 0.0000671 au
Point group: D3H
Sb-F equitorial bond distance: 1.90Å
Sb-F axial bond distance: 1.91Å
F-Sb-F equitorial bond angle: 120°
F-Sb-F axial bond angle: 180°
NH3 |
The optimisation file for SbF5 can be found here
Checking the optimization of SbF5
Item Value Threshold Converged?
Maximum Force 0.000143 0.000450 YES
RMS Force 0.000059 0.000300 YES
Maximum Displacement 0.000589 0.001800 YES
RMS Displacement 0.000248 0.001200 YES
Predicted change in Energy=-1.693505D-07
Optimization completed.
-- Stationary point found.
See 'Checking the opimisation of NH3' for an explanation of this table.
Frequency and charge analysis for SbF5
Mode Frequency Infrared 1 94.3 0.6211 2 94.3 0.6211 3 237 63.8399 4 237 63.8390 5 247 0.0000 6 247 0.0000 7 258 65.7404 8 572 0.0000 9 587 0.0000 10 641 69.9319 11 647 60.5214 12 647 60.5214
| Wavenumber (cm-1) | symmetry | inensity (arbitrary units) |
|---|---|---|
| 94.3 | E' | 0.6211 |
| 94.3 | E' | 0.6211 |
| 237 | E' | 68.8399 |
| 237 | E' | 63.8390 |
| 247 | E | 0.000 |
| 247 | E | 0.000 |
| 258 | A2 | 65.7404 |
| 572 | A1' | 0.000 |
| 587 | A1' | 0.000 |
| 641 | A2 | 69.9319 |
| 647 | E' | 60.5214 |
| 647 | E' | 60.5214 |
See the 'Frequency and charge analysis for N2' for an explanation as to why at some frequencies of vibration there is no infrared value present.
The relative charge on the Sb atom is 3.040. The relative charge on each of the equitorial F atoms is -0.606, whilst the relative charge on each of the axial F atoms is -0.612.
Marking
Note: All grades and comments are provisional and subject to change until your grades are officially returned via blackboard. Please do not contact anyone about anything to do with the marking of this lab until you have recieved your grade from blackboard.
Wiki structure and presentation 1/1
Is your wiki page clear and easy to follow, with consistent formatting?
YES
Do you effectively use tables, figures and subheadings to communicate your work?
YES
NH3 0.5/1
Have you completed the calculation and given a link to the file?
YES
Have you included summary and item tables in your wiki?
YES
Have you included a 3d jmol file or an image of the finished structure?
YES
Have you included the bond lengths and angles asked for?
YES I think you have made a mistake here as the angle should be much larger than 37! I think you probably did a H-H-N angle instead.
Have you included the “display vibrations” table?
YES
Have you added a table to your wiki listing the wavenumber and intensity of each vibration?
YES
Did you do the optional extra of adding images of the vibrations?
NO
Have you included answers to the questions about vibrations and charges in the lab script?
You have answered most of the vibration questions well - good job!
However due to the low intensity of vibrations 4, 5 and 6 you only see two peaks in the IR spectrum.
Also you forgot to answer the question about the charges in the script: "Write a sentence saying what charge (positive or negative) you would expect for N and H and why"
N2 and H2 0/0.5
Have you completed the calculations and included all relevant information? (summary, item table, structural information, jmol image, vibrations and charges)
YES - this is well structured and the explanation of charges is good.
However you have given a bond angle of 180 for N2 and H2, there are no bond angles in diatomic molecules. Bond angles involve exactly 3 atoms.
Crystal structure comparison 0.5/0.5
Have you included a link to a structure from the CCDC that includes a coordinated N2 or H2 molecule?
YES
Have you compared your optimised bond distance to the crystal structure bond distance?
YES - good details in the explanation.
Haber-Bosch reaction energy calculation 1/1
Have you correctly calculated the energies asked for? ΔE=2*E(NH3)-[E(N2)+3*E(H2)]
YES
Have you reported your answers to the correct number of decimal places?
YES
Do your energies have the correct +/- sign?
YES
Have you answered the question, Identify which is more stable the gaseous reactants or the ammonia product?
YES
Your choice of small molecule 5/5
Have you completed the calculation and included all relevant information?
YES
Have you added information about MOs and charges on atoms?
YES your explanations about the MOs were very detailed, and you showed good understanding of bonding and antibonding interactions, well done.
Note this phrase: "4 lobes are present as the spins do not overlap." is not correct and doesn't really make sense to me. The different colours in the MOs are phases and are not to do with spin.
Independence 1/1
If you have finished everything else and have spare time in the lab you could: Check one of your results against the literature, or Do an extra calculation on another small molecule, or
YES
Do some deeper analysis on your results so far